Chemistry - Nomenclature of coordination compounds | 12th Chemistry : UNIT 5 : Coordination Chemistry
Chapter: 12th Chemistry : UNIT 5 : Coordination Chemistry
Nomenclature of coordination compounds
Nomenclature
of coordination compounds
In the earlier days, the
compounds were named after their discoverers. For example, K[PtCl3(C2H4)]
was called Zeise’s salt and [Pt(NH3)4][PtCl4]
is called Magnus’s green salt etc...
There are numerous
coordination compounds that have been synthesised and characterised. The
International Union of Pure and Applied Chemistry (IUPAC) has developed an
elaborate system of nomenclature to name them systematically. The guidelines
for naming coordination compounds based on IUPAC recommendations (2005) are as
follows:
The cation is named
first, followed by the anion regardless of weather the ion is simple or
complex. For example
·
In K4[Fe(CN)6], the cation K+ is
named first followed by[Fe(CN)6]4-.
·
In [Co(NH3)6]Cl3, the complex
cation [Co(NH3)6]3+ is named first followed by
the anion Cl-
·
In [Pt(NH3)4][PtCl4], the complex
cation [Pt(NH3)4]2+is named first followed by
the complex anion [PtCl4]2-
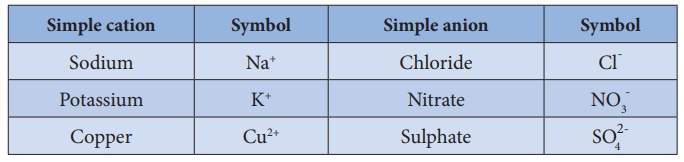
2. The simple ions are
named as in other ionic compounds. For example,
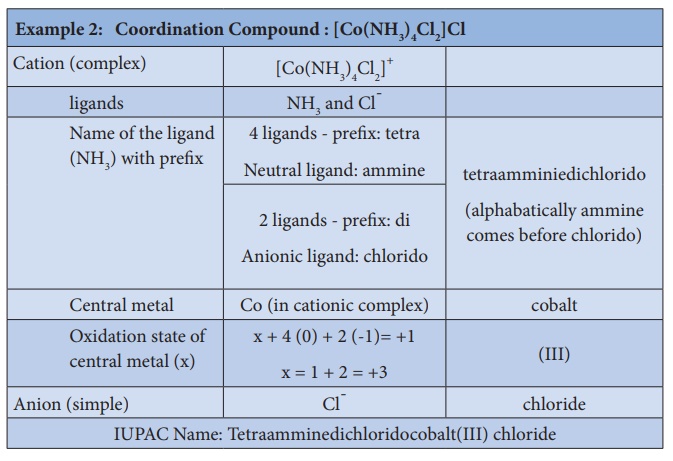
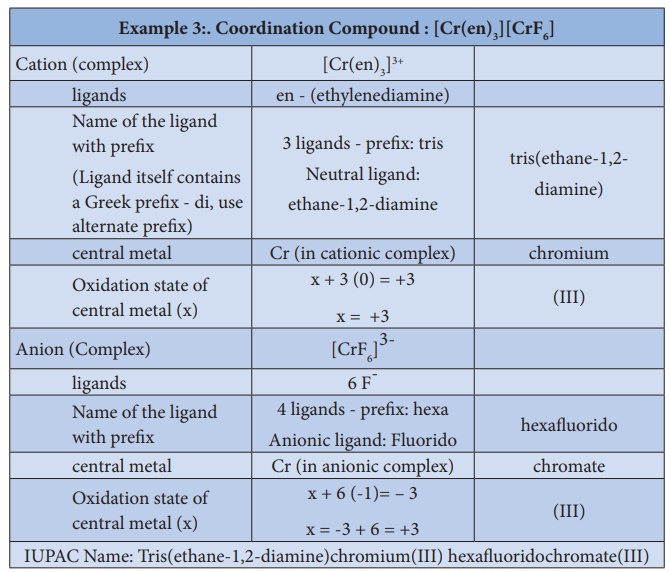
3. To name a complex ion, the ligands are named first followed by
the central metal atom/ion. When a complex ion contains more than one kind of
ligands they are named in alphabetical order.
a. Naming the ligands:
i. The name of anionic ligands ends with the letter 'o' and the
cationic ligand ends with 'ium'. The neutral ligands are usually called with
their molecular names with fewer exceptions namely, H2O (aqua), CO (carbonyl),
NH3 (ammine) and NO (nitrosyl).
ii. A κ-term is used to
denote an ambidendate ligand in which more than one coordination mode is
possible. For example, the ligand thiocyanate can bind to the central atom/
ion, through either the sulfur or the nitrogen atom. In this ligand, if sulphur
forms a coordination bond with metal then the ligand is named thiocyanato-κS
and if nitrogen is involved, then it is named thiocyanato-κN.
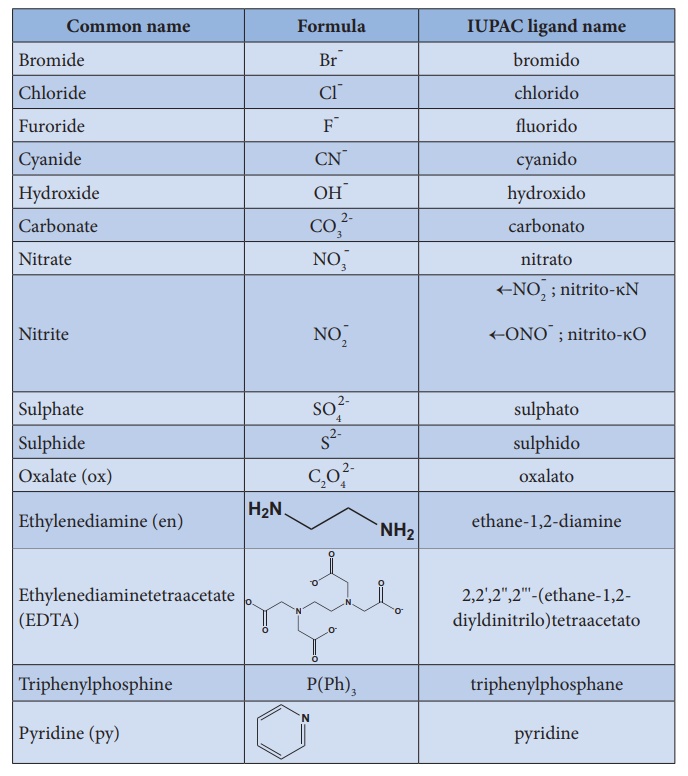
iii. If the coordination entity contains more than one ligand of a
particular type, the multiples of ligand (2, 3, 4 etc...) is indicated by
adding appropriate Greek prefixes (di, tri, tetra, etc...) to the name of the
ligand. If the name of a ligand itself contains a Greek prefix (eg.
ethylenediamine), use an alternate prefixes (bis, tris, tetrakis etc..) to
specify the multiples of such ligands. These numerical prefixes are not taken
into account for alphabetising the name of ligands.
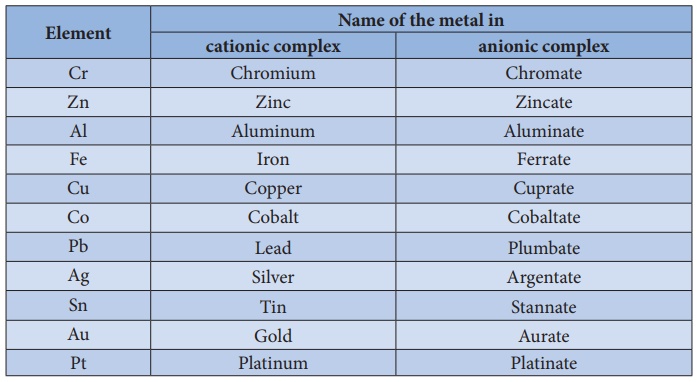
b. Naming
the central metal:
In cationic/neutral
complexes, the element name is used as such for naming the central metal atom/ion,
whereas, a suffix 'ate' is used along with the element name in anionic
complexes. The oxidation state of the metal is written immediately after the
metal name using roman numerals in parenthesis.
Naming of coordination
compounds using IUPAC guidelines.
Example 1:
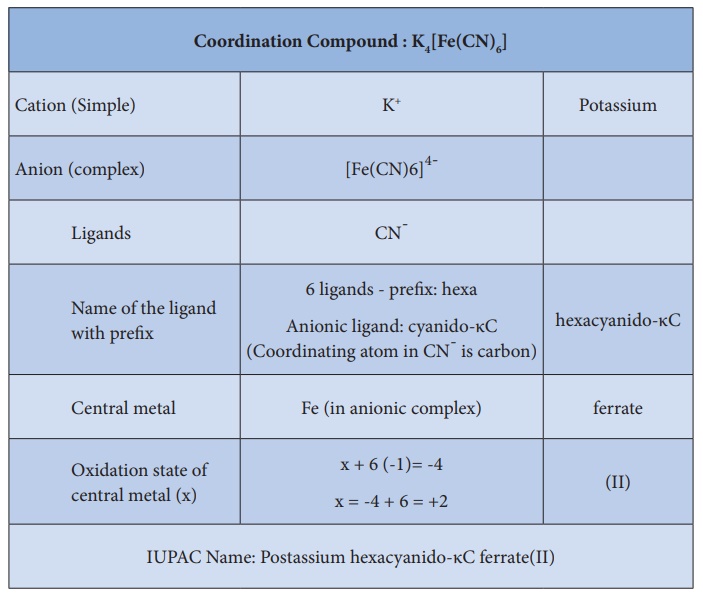
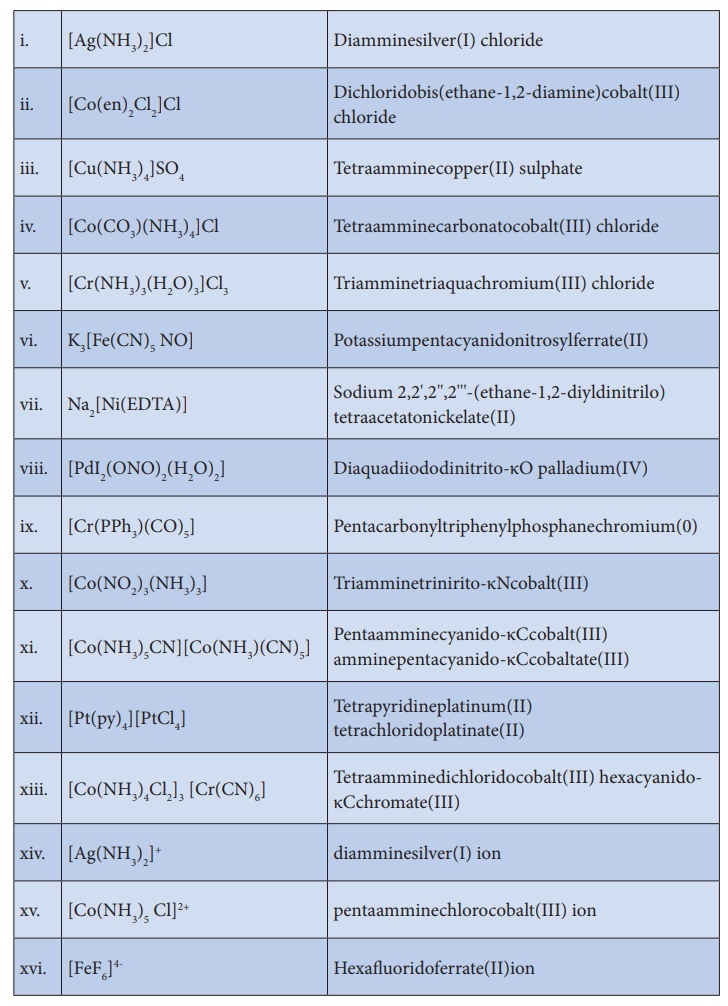
More examples with
names are given in the list below for better understanding of IUPAC
Nomenclature:
Related Topics