Chemistry - Isomerism in coordination compounds | 12th Chemistry : UNIT 5 : Coordination Chemistry
Chapter: 12th Chemistry : UNIT 5 : Coordination Chemistry
Isomerism in coordination compounds
Isomerism
in coordination compounds
We have already learnt
the concept of isomerism in the context of organic compounds, in the previous
year chemistry classes. Similarly, coordination compounds also exhibits
isomerism. Isomerism is the phenomenon in which more than one coordination
compounds having the same molecular formula have different physical and
chemical properties due to different arrangement of ligands around the central
metal atom. The following flow chart gives an overview of the common types of
isomerism observed in coordination compounds,

Structural isomers
The coordination
compounds with same formula, but have different connections among their
constituent atoms are called structural isomers or constitutional isomers. Four
common types of structural isomers are discussed below.
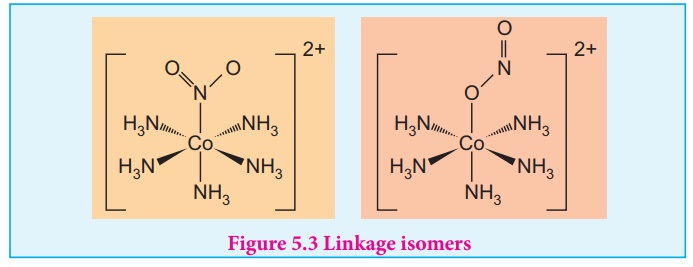
Linkage isomers:
This type of isomers
arises when an ambidentate ligand is bonded to the central metal atom/ion
through either of its two different donor atoms. In the below mentioned
examples, the nitrite ion is bound to the central metal ion Co3+ through a
nitrogen atom in one complex,and through oxygen atom in other complex.
[Co(NH3)5(NO2)]2+
Coordination isomers:
This type of isomers
arises in the coordination compounds having both the cation and anion as
complex ions. The interchange of one or more ligands between the cationic and
the anionic coordination entities result in different isomers.
For example, in the
coordination compound, [Co(NH3)6][Cr(CN)6] the
ligands ammonia and cyanide were bound respectively to cobalt and chromium
while in its coordination isomer [Cr(NH3)6][Co(CN)6]
they are reversed.
Some more examples for coordination isomers
1. [Cr(NH3)5CN][Co(NH3)(CN)5]
and [Co(NH3)5CN][Cr(NH3)(CN)5]
2. [Pt(NH3)4][Pd(Cl)4] and [Pd(NH3)4][Pt(Cl)4]
Ionisation isomers:
This type of isomers
arises when an ionisable counter ion (simple ion) itself can act as a ligand.
The exchange of such counter ions with one or more ligands in the coordination
entity will result in ionisation isomers. These isomers will give different
ions in solution. For example, consider the coordination compound [Pt(en)2Cl2]Br2.
In this compound, both Br-and Cl- have the ability to act
as a ligand and the exchange of these two ions result in a different isomer
[Pt(en)2Br2]Cl2. In solution the first
compound gives Br - ions while the later gives Cl-ions
and hence these compounds are called ionisaiton isomers.
Some more example for
the isomers,
1. [Cr(NH3)4ClBr]NO2
and [Cr(NH3)4Cl NO2]Br
2. [Co(NH3)4Br2]Cl
and [Co(NH3)4Cl Br]Br
Solvate isomers.
The exchange of free
solvent molecules such as water , ammonia, alcohol etc.. in the crystal lattice
with a ligand in the coordination entity will give different isomers. These
type of isomers are called solvate isomers. If the solvent molecule is water,
then these isomers are called hydrate isomers. For example, the complex with
chemical formula CrCl3.6H2O has three hydrate isomers as
shown below.

Stereoisomers:
Similar to organic
compounds, coordination compounds also exhibit stereoisomerism. The
stereoisomers of a coordination compound have the same chemical formula and
connectivity between the central metal atom and the ligands. But they differ in
the spatial arrangement of ligands in three dimensional space. They can be
further classified as geometrical isomers and optical isomers.
Geometrical isomers:
Geometrical isomerism
exists in heteroleptic complexes due to different possible three dimensional
spatial arrangements of the ligands around the central metal atom. This type of
isomerism exists in square planer and octahedral complexes.
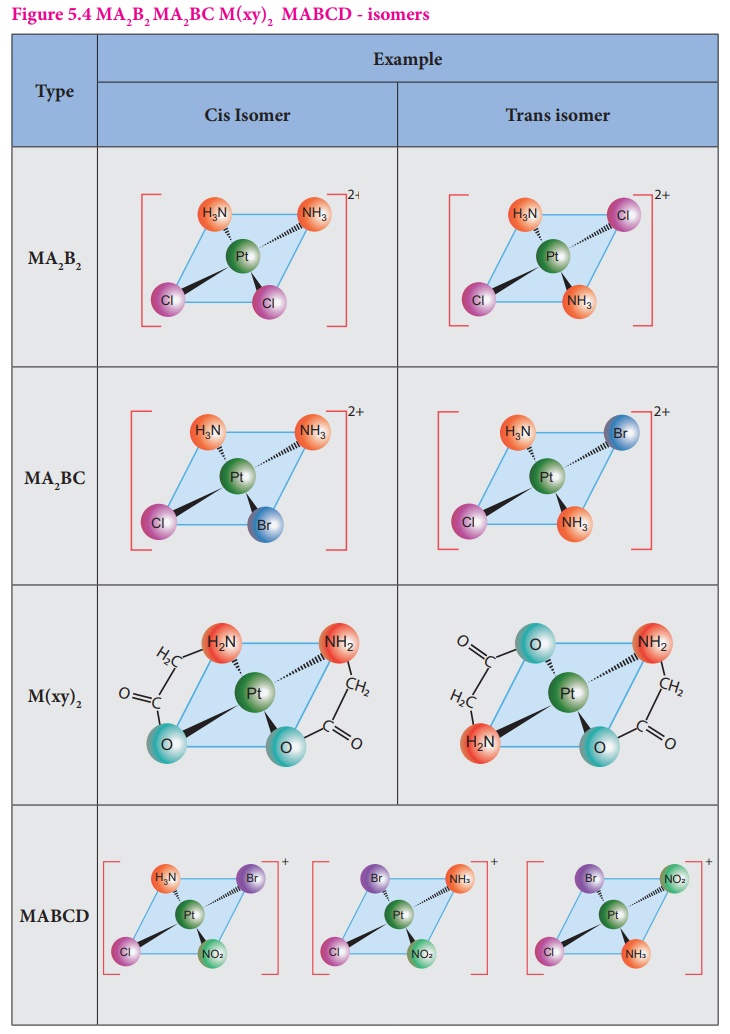
In square planar
complexes of the form [MA2B2]n± and [MA2BC]n±
(where A, B and C are mono dentate ligands and M is the central metal
ion/atom), Similar groups (A or B) present either on same side or on the
opposite side of the central metal atom (M) give rise to two different
geometrical isomers, and they are called, cis and trans isomers respectively.
The square planar
complex of the type [M(xy)2]n± where xy is a bidentate
ligand with two different coordinating atoms also shows cis-trans
isomerism. Square planar complex of the form [MABCD]n± also shows
geometrical isomerism. In this case, by considering any one of the ligands (A,
B, C or D) as a reference, the rest of the ligands can be arranged in three
different ways leading to three geometrical isomers.
Octahedral complexes:
Octahedral complexes of
the type [MA2B4]n±, [M(xx)2B2]n±
shows cis-trans isomerism. Here A and B are monodentate ligands and xx
is bidentate ligand with two same kind of donor atoms. In the octahedral
complex, the position of ligands is indicated by the following numbering
scheme.
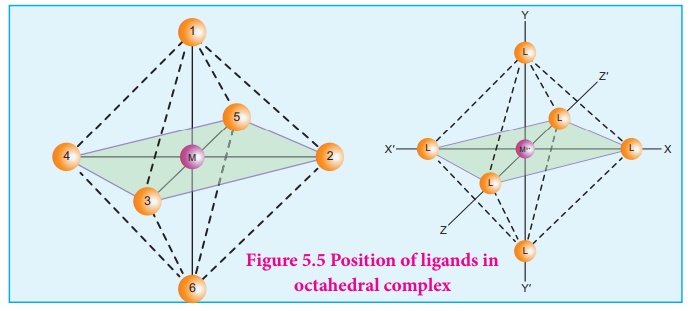
In the above scheme, the
positions (1,2), (1,3), (1,4), (1,5), (2,3), (2,5), (2,6), (3,4), (3,6), (4,5),
(4,6), and (5,6) are identical and if two similar groups are present in any one
of these positions, the isomer is referred as a cis isomer. Similarly,
positions (1,6), (2,4), and (3,5) are identical and if similar ligands are
present in these positions it is referred as a trans-isomer.
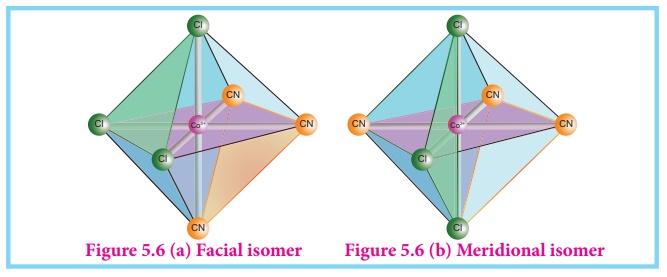
Octahedral complex of
the type [MA3B3]n± also shows geometrical
isomerism. If the three similar ligands (A) are present in the corners of one
triangular face of the octahedron and the other three ligands (B) are present
in the opposing triangular face, then the isomer is referred as a facial isomer
(fac isomer)- Figure 5.6 (a).
If the three similar
ligands are present around the meridian which is an imaginary semicircle from
one apex of the octahedral to the opposite apex as shown in the figure 5.6(b),
the isomer is called as a meridional isomer (mer isomer). This is called
meridional because each set of ligands can be regarded as lying on a meridian
of an octahedron.
As the number of
different ligands increases, the number of possible isomers also increases. For
the octahedral compled of the type [MABCDEF]n±, where A, B, C, D, E
and F are monodentate ligands, fifteen different orientation are possible
corresponding to 15 geometrical isomers. It is difficult to generate all the
possible isomers.
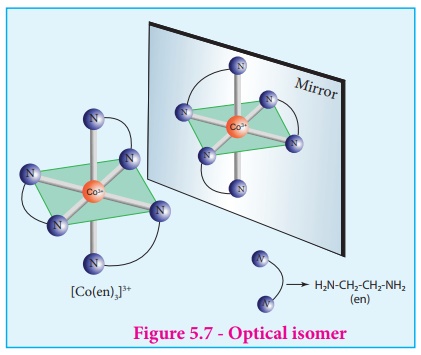
Optical Isomerism
Coordination compounds
which possess chairality exhibit optical isomerism similar to organic
compounds. The pair of two optically active isomers which are mirror images of each
other are called enantiomers. Their solutions rotate the plane of the plane
polarised light either clockwise or anticlockwise and the corresponding isomers
are called 'd' (dextro rotatory) and 'l' (levo rotatory) forms respectively.
The octahedral complexes
of type [M(xx)3]n±,
[M(xx)2AB]n± and [M(xx)2B2]n±
exhibit optical isomerism.
Examples:
The optical isomers of
[Co(en)3]3+ are shown in figure 5.7.
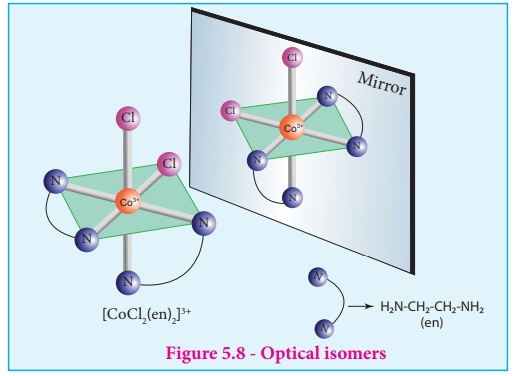
The coordination complex
[CoCl 2(en)2]+ has three isomers, two
optically active cis forms and one optically inactive trans form. These
structures are shown below.
Related Topics