Chapter: 12th Chemistry : UNIT 5 : Coordination Chemistry
Coordination Chemistry: Choose the correct answer
Coordination Chemistry
Choose the correct answer:
1. The sum of primary valance and secondary valance of the metal M in the complex [ M ( en)2 ( Ox)] Cl is L
a) 3
b) 6
c) -3
d) 9
Solution
In the complex [M (en)2 (Ox)] Cl For the central metal ion M3+
The primary valance is = +3
The secondary valance = 6
sum of primary valance and secondary valance = 3+6 = 9
Answer : option (d)
2. An excess of silver nitrate is added to 100ml of a 0.01M solution of pentaaqua chloride chromium(III)chloride. The number of moles of AgCl precipitated would be
a) 0.02
b) 0.002
c) 0.01
d) 0.2
Solution
The complex is [M(H2O)5 Cl] Cl2
1000 ml of 1M solution of the complex gives 2 moles of Cl− ions
1000 ml of 0.01M solution of the complex will give
[100 ml x 0.01M x 2Cl-] / [1000 ml x 1M] = 0.002 moles of C− ions
Answer : option (b)
3. A complex has a molecular formula MSO4 Cl. 6H2O .The aqueous solution of it gives white precipitate with Barium chloride solution and no precipitate is obtained when it is treated with silver nitrate solution. If the secondary valence of the metal is six, which one of the following correctly represents the complex?
a) [M ( H2O)4 Cl] SO4 .2H2O
b) [M ( H2O)6 ]SO4
c) [M ( H2O)5 Cl] SO4 .H2O
d) [M ( H2O)3 Cl ] SO4 .3H2O
Solution
Molecular formula: MSO4Cl. 6H2O .
Formation of white precipitate with Barium chloride indicates that SO42- ions are outside the coordination sphere, and no precipitate with AgNO3 solution indicates that the Cl− ions are inside the coordination sphere.Since the coordination number of M is 6, Cl− and 5 H2O are ligands, remaining 1 H2O molecular and SO42- are in the outer coordination sphere.
Answer : option (c)
4. Oxidation state of Iron and the charge on the ligand NO in [ Fe ( H2O)5 NO] SO4 are
a) +2 and 0 respectively
b) +3 and 0 respectively
c) +3 and -1 respectively
d) +1 and +1 respectively
Solution
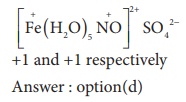
5. As per IUPAC guidelines, the name of the complex [Co( en)2 ( ONO)Cl ] Cl is
a) chlorobisethylenediaminenitritocobalt(III) chloride
b) chloridobis(ethane-1,2-diamine)nitro k-Ocobaltate(III) chloride
c) chloridobis(ethane-1,2-diammine)nitrito k -Ocobalt(II) chloride
d) chloridobis(ethane-1,2-diamine)nitro k -Ocobalt(III) chloride
6. IUPAC name of the complex K3 [Al ( C2O4 )3 ] is
a) potassiumtrioxalatoaluminium(III)
b) potassiumtrioxalatoaluminate(II)
c) potassiumtrisoxalatoaluminate(III)
d) potassiumtrioxalatoaluminate(III)
7. A magnetic moment of 1.73BM will be shown by one among the following
a) TiCl4
b) [CoCl6] 4-
c) [Cu (NH3)4]2+
d) [Ni (CN)4 ]2-
Solution

8. Crystal field stabilization energy for high spin d5 octahedral complex is
a) – 0.6 ∆0.
b) 0
c) 2 (P −∆0)
d) 2 (P+ ∆0)
Solution
The electronic configuration t2g3, eg2
[3x(-0.4) + 2(0.6)]Δ0
[-1.2 + 1.2]Δ0 =0
Answer : option(b)
9. In which of the following coordination entities the magnitude of Δ0 will be maximum?
a) [Co (CN)6]3-
b) [ Co(C2O4)3]3-
c) [Co(H2O)6]3+
d) [Co(NH3)]3+
Solution
In all the complexes, the central metal ion is Co3+, among the given ligands CN− is the strongest ligand, which causes large crystal field splitting i.e maximum ∅0
10. Which one of the following will give a pair of enantiomorphs?
a) [Cr(NH3)6 ] [Co(CN)6]
b) [Co( en)2 Cl2]Cl
c) [Pt (NH3)4 ] [PtCl4]
d) [Co(NH3)2 Cl2]?
Solution
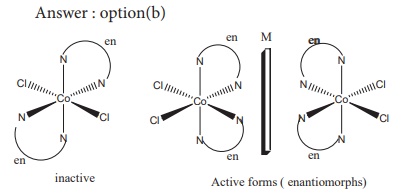
Complexes given in other options (a), (c) and (d) have symmetry elements and hence they are optically inactive.
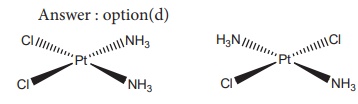
11. Which type of isomerism is exhibited by Pt ( NH3)2 Cl2 ?
a) Coordination isomerism
b) Linkage isomerism
c) Optical isomerism
d) Geometrical isomerism
Solution
12. How many geometrical isomers are possible for [ Pt(Py)(NH3)(Br)(Cl)] ?
a) 3
b) 4
c) 0
d) 15
Solution
Three isomers. If we consider any one of the ligands as reference ( say Py), the arrangement of other three ligands ( NH3, Br and Cl‑ ) with respect to (Py) gives three geometrical isomers.
13. Which one of the following pairs represents linkage isomers?
a) [Cu ( NH3)4] [PtCl4] and [Pt ( NH3)4] [CuCl4]
b) [Co ( NH3)5( NO3)] SO4 and [ Co (NH3)5(ONO)]
c) [Co ( NH3 )4 ( NCS)2] Cl and [Co ( NH3)4 ( SCN)2] Cl
d) both (b) and (c)
Solution
(a) coordination isomers
(b) no isomerism ( different molecular formula)
(c) ← NCS , ← SCN coordinating atom differs : linkage isomers
14. Which kind of isomerism is possible for a complex [Co ( NH3)4 Br2] Cl ?
a) geometrical and ionization
b) geometrical and optical
c) optical and ionization
d) geometrical only
Solution
For [ MA4 B2 ]n+ complexes-geometrical isomerism is possible
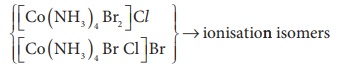
15. Which one of the following complexes is not expected to exhibit isomerism?
a) [Ni(NH3)4(H2O)2]2+
b) [Pt(NH3)2Cl2]
c) [Co(NH3)5SO4]Cl
d) [Fe(en)3]3+
Solution
Option (a) and (b) – geometrical isomerism is possible
Option (c) – ionization isomerism is possible
Option (d) – no possibility to show either constitutional isomerism or stereo isomerism
16. A complex in which the oxidation number of the metal is zero is
a) K4[Fe(CN)6]
b) [Fe(CN)3(NH3)3]
c) [Fe(CO)5]
d) both (b) and (c)
Solution
(a) Fe2+
(b) Fe3+
(c) Fe0
17. Formula of tris(ethane-1,2-diamine)iron(II)phosphate
a) [Fe(CH3-CH(NH2)2)3](PO4)3
b) [Fe(H2N-CH2-CH2-NH2)3)](PO4)
c) [Fe(H2N-CH2-CH2-CH2-NH2)3](PO4)2
d) [Fe(H2N-CH2-CH2-NH2)3]3(PO4)2
Solution
[Fe (en )3]2+ (PO43-)
18. Which of the following is paramagnetic in nature?
a) [Zn(NH3)4]2+
b) [Co(NH3)6]3+
c) [Ni(H2O)6]2+
d) [Ni(CN)4]2-
Solution
(a) Zn2+ (d10 ⟹ diamagnetic)
(b) Co3+ (d6 Low spin ⟹ t2g6, eg0 diamagnetic)
(c) Ni2+ (d8 Low spin ⟹ t2g6, eg2 paramagnetic)
(d) [Ni(CN)4]2- (dsp2 ; square planar, diamagnetic)
19. Fac-mer isomerism is shown by
a) [Co(en)3]3+
b) [Co(NH3)4(Cl)2] +
c) [Co(NH3)3(Cl)2]
d) [Co(NH3)5Cl]SO4
Solution
[Co (NH3)3(Cl)3]
20. Choose the correct statement.
a) Square planar complexes are more stable than octahedral complexes
b) The spin only magnetic moment of [Cu ( Cl)4 ]2− is 1.732 BM and it has square planar structure.
c) Crystal field splitting energy (∆0) of [FeF6]4- is higher than the (∆0) of [Fe (CN)6]4-
d) crystal field stabilization energy of [V(H2O)6]2+ is higher than the crystal field stabilization of [Ti (H2O)6]2+
Solution
V2+ ( t2g3, eg0 ; CFSE = 3 x (-0.4)Δ0 = -1.2 Δ0)
Ti2+ ( t2g2, eg0 ; CFSE = 2 x (-0.4)Δ0 = -0.8 Δ0)
Statements given in option (a) ,(b), and (c) are wrong.
The current statements are
(a) since, the crystal field stabilization is more in octahedral field , octahedral complexes are more stable than square planar complexes.
(b) 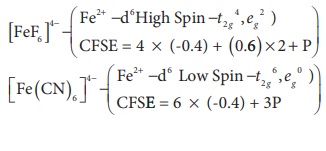
PTA Question Oneword:
1.
According spectrochemical series which of the following ligand produces
strongest field and cause maximum splitting?
a)
F−
b) CO
c)
H2O
d)
Cl −
Answer: b)
2.
What kind of isomerism is possible for a complex [Co(NH3)4Br2]Cl?
a) geometrical and Ionisation
b)
geometrical and Optical
c)
Optical and Ionisation
d)
Geometrical only
Answer: a)
3.
Among the following complexes, the one which shows zero crystal field
stabilization energy (CFSE) is?
a) [Fe(H2O)6]3+
b)
[Mn(H2O)6]3+
c)
[Co(H2O)6]3+
d)
[Co(H2O)6]2+
Answer: a)
Solution: Fe3+
− d5 t2g3eg2
=
(−3 × 0.4 + 2 × 0.6) ∆0
=
(−1.2 + 1.2) ∆0 = 0
Related Topics