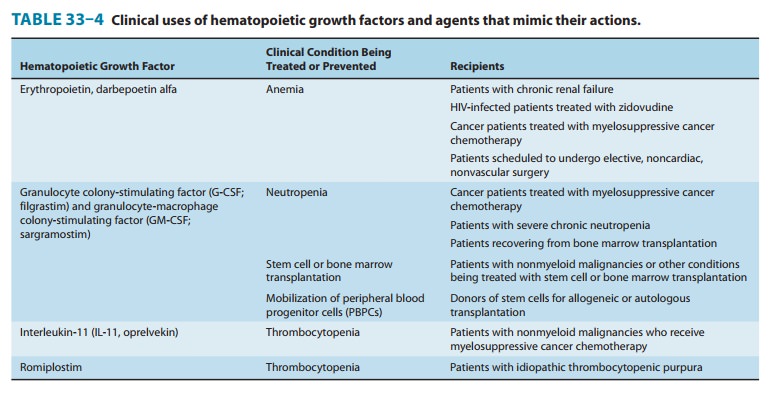
Chapter: Basic & Clinical Pharmacology : Agents Used in Anemias; Hematopoietic Growth Factors
Myeloid Growth Factors
MYELOID GROWTH FACTORS
Chemistry & Pharmacokinetics
G-CSF and GM-CSF, the two
myeloid growth factors currentlyavailable for clinical use, were originally
purified from cultured human cell lines (Table 33–4). Recombinant human G-CSF (rHuG-CSF; filgrastim) is produced in a
bacterial expression system. It is a nonglycosylated peptide of 175 amino
acids, with a molecular weight of 18 kDa. Recombinant human GM-CSF (rHuGM-CSF; sargramostim) is produced in
a yeast expression system. It is a partially glycosylated peptide of 127 amino
acids, with three molecular species with molecular weights of 15,500; 15,800;
and 19,500. These preparations have serum half-lives of 2–7 hours after
intravenous or subcutaneous administration. Pegfilgrastim, a covalent conjugation product of filgrastim and
aform of polyethylene glycol, has a much longer serum half-life than
recombinant G-CSF, and it can be injected once per myelo-suppressive
chemotherapy cycle instead of daily for several days. Lenograstim, used widely in Europe, is a glycosylated form ofrecombinant
G-CSF.
Pharmacodynamics
The myeloid growth
factors stimulate proliferation and differen-tiation by interacting with
specific receptors found on myeloid progenitor cells. Like the erythropoietin
receptor, these receptors are members of the JAK/STAT superfamily . G-CSF
stimulates proliferation and differentiation of progenitors already committed
to the neutrophil lineage. It also activates the phagocytic activity of mature
neutrophils and prolongs their survival in the circulation. G-CSF also has a
remarkable ability to mobilize hematopoietic stem cells, ie, to increase their
concentra-tion in peripheral blood. This biologic effect underlies a major
advance in transplantation—the use of peripheral
blood stemcells (PBSCs) rather than bone marrow stem cells for
autologousand allogeneic hematopoietic stem cell transplantation .
GM-CSF has broader
biologic actions than G-CSF. It is a multipotential hematopoietic growth factor
that stimulates prolif-eration and differentiation of early and late
granulocytic progeni-tor cells as well as erythroid and megakaryocyte
progenitors
Erythropoietin
has been used successfully to offset the anemia produced by zidovudine
treatment in patients with HIV infection and in the treatment of the anemia of
prematurity. It can also be used to reduce the need for transfusion in
high-risk patients undergoing elective, non-cardiac, nonvascular surgery.
Erythropoietin
is one of the drugs banned by the International Olympic Committee. The use of
erythropoietin by athletes is based on their hope that increased red blood cell
concentration will increase oxygen delivery to muscles and improve performance.
Toxicity
The most common
adverse effects of erythropoietin are hyperten-sion and thrombotic
complications. In March 2007, the FDA issued a warning that patients with
chronic renal failure or cancer whose serum hemoglobin is raised to more than
12 g/dL with an ESA face a greater risk of a thrombotic event or, in patients
with advanced head and neck cancers, faster tumor growth. The warn-ing was
primarily based on clinical trial data from patients with chronic kidney
disease indicating an increased rate of mortality and cardiovascular events
(stroke, myocardial infarction, worsen-ing congestive heart failure, and
hypertension) in patients dosed with an ESA to a target hemoglobin level of
12–16 g/dL or dosed to maintain a normal hematocrit (42%) versus a lower target
hematocrit of 30%. In addition, a meta-analysis of 51 placebo-controlled trials
of ESAs in cancer patients reported an increased rate of all-cause mortality
and venous thrombosis in those receiving an ESA. Based on the accumulated
evidence, it is recommended that the hemoglobin level not exceed 12 g/dL in
patients with chronic kidney disease receiving an ESA, and that ESAs be used
conservatively in cancer patients (eg, when Like G-CSF, GM-CSF also stimulates
the function of mature neutrophils. GM-CSF acts together with interleukin-2 to
stimu-late T-cell proliferation and appears to be a locally active factor at
the site of inflammation. GM-CSF mobilizes peripheral blood stem cells, but it
is significantly less efficacious and more toxic than G-CSF in this regard.
Clinical Pharmacology
A. Cancer Chemotherapy-Induced Neutropenia
Neutropenia is a
common adverse effect of the cytotoxic drugs used to treat cancer and increases
the risk of serious infection in patients receiving chemotherapy. Unlike the
treatment of anemia and thrombocytopenia, transfusion of neutropenic patients
with granulocytes collected from donors is performed rarely and with limited
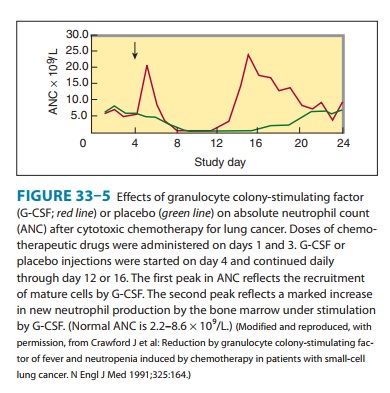
success. The introduction of G-CSF in 1991 represented a milestone in the
treatment of chemotherapy-induced neutrope-nia. This growth factor dramatically
accelerates the rate of neutro-phil recovery after dose-intensive
myelosuppressive chemotherapy (Figure 33–5). It reduces the duration of
neutropenia and usually raises the nadir count, the lowest neutrophil count
seen following a cycle of chemotherapy.The ability of G-CSF to increase neutrophil
counts after myelosuppressive chemotherapy is nearly universal, but its impact
on clinical outcomes is more variable. Many, but not all, clinical trials and
meta-analyses have shown that G-CSF reduces episodes of febrile neutropenia,
requirements for broad-spectrum antibiot-ics, infections, and days of
hospitalization. Clinical trials have not shown improved survival in cancer
patients treated with G-CSF. Clinical guidelines for the use of G-CSF after
cytotoxic chemo-therapy recommend reserving G-CSF for patients at high risk for
febrile neutropenia based on age, medical history, and disease characteristics;
patients receiving dose-intensive chemotherapy
regimens
that carry a greater than 40% risk of causing febrile neutropenia; patients
with a prior episode of febrile neutropenia after cytotoxic chemotherapy;
patients at high risk for febrile neutro-penia; and patients who are unlikely
to survive an episode of febrile neutropenia. Pegfilgrastim is an alternative
to G-CSF for prevention of chemotherapy-induced febrile neutropenia.
Pegfilgrastim can be administered once per chemotherapy cycle, and it may
shorten the period of severe neutropenia slightly more than G-CSF.
Like
G-CSF and pegfilgrastim, GM-CSF also reduces the dura-tion of neutropenia after
cytotoxic chemotherapy. It has been more difficult to show that GM-CSF reduces
the incidence of febrile neutropenia, probably because GM-CSF itself can induce
fever. In the treatment of chemotherapy-induced neutropenia, G-CSF, 5 mcg/kg/d,
or GM-CSF, 250 mcg/m2/d, is
usually started within 24–72 hours after completing chemotherapy and is
continued until the absolute neutrophil count is greater than 10,000 cells/μL.
Pegfilgrastim is given as a single dose of 6 mg.
The
utility and safety of the myeloid growth factors in the postchemotherapy
supportive care of patients with acute myeloid leukemia (AML) have been the
subject of a number of clinical trials. Because leukemic cells arise from
progenitors whose proliferation and differentiation are normally regulated by
hematopoietic growth factors, including GM-CSF and G-CSF, there was concern
that myeloid growth factors could stimulate leukemic cell growth and increase
the rate of relapse. The results of randomized clinical trials suggest that
both G-CSF and GM-CSF are safe following induction and consolidation treatment
of myeloid and lymphoblastic leuke-mia. There has been no evidence that these
growth factors reduce the rate of remission or increase relapse rate. On the
contrary, the growth factors accelerate neutrophil recovery and reduce
infection rates and days of hospitalization. Both G-CSF and GM-CSF have FDA
approval for treatment of patients with AML.
B. Other Applications
G-CSF and GM-CSF have
also proved to be effective in treating the neutropenia associated with congenital neutropenia, cyclicneutropenia,
myelodysplasia, and aplastic anemia.
Manypatients with these disorders respond with a prompt and some-times
dramatic increase in neutrophil count. In some cases, this results in a
decrease in the frequency of infections. Because neither G-CSF nor GM-CSF
stimulates the formation of erythrocytes and platelets, they are sometimes
combined with other growth factors for treatment of pancytopenia.
The
myeloid growth factors play an important role in autolo-gous stem cell transplantation for patients undergoing
high-dosechemotherapy. High-dose chemotherapy with autologous stem cell support
is increasingly used to treat patients with tumors that are resistant to
standard doses of chemotherapeutic drugs. The high-dose regimens produce
extreme myelosuppression; the myelosuppression is then counteracted by reinfusion
of the patient’s own hematopoietic stem cells (which are collected prior to
chemotherapy). The administration of G-CSF or GM-CSF early after autologous
stem cell transplantation reduces the time to engraftment and to recovery from
neutropenia in patients receiving stem cells obtained either from bone marrow
or from peripheral blood. These effects are seen in patients being treated for
lymphoma or for solid tumors. G-CSF and GM-CSF are also used to support
patients who have received allogeneic bone mar-row transplantation for
treatment of hematologic malignancies or bone marrow failure states. In this
setting, the growth factors speed the recovery from neutropenia without
increasing the inci-dence of acute graft-versus-host disease.
Perhaps the most
important role of the myeloid growth factors in transplantation is for
mobilization of PBSCs. Stem cells col-lected from peripheral blood have nearly
replaced bone marrow as the hematopoietic preparation used for autologous and
allogeneic transplantation. The cells can be collected in an outpatient setting
with a procedure that avoids much of the risk and discomfort of bone marrow
collection, including the need for general anesthesia. In addition, there is
evidence that PBSC transplantation results in more rapid engraftment of all
hematopoietic cell lineages and in reduced rates of graft failure or delayed
platelet recovery.
G-CSF
is the cytokine most commonly used for PBSC mobi-lization because of its
increased efficacy and reduced toxicity compared with GM-CSF. To mobilize stem
cells for autologous transplantation, donors are given 5–10 mcg/kg/d
subcutaneously for 4 days. On the fifth day, they undergo leukapheresis. The
suc-cess of PBSC transplantation depends on transfusion of adequate numbers of
stem cells. CD34, an antigen present on early pro-genitor cells and absent from
later, committed, cells, is used as a marker for the requisite stem cells. The
goal is to infuse at least 5 × 106
CD34 cells/kg; this number of CD34 cells usually results in prompt and durable
engraftment of all cell lineages. It may take several separate leukaphereses to
collect enough CD34 cells, espe-cially from older patients and patients who
have been exposed to radiation therapy or chemotherapy.
For
patients with multiple myeloma or non-Hodgkin’s lym-phoma who respond
suboptimally to G-CSF alone, the novel hematopoietic stem cell mobilizer plerixafor can be added to G-CSF.
Plerixafor is a bicyclam molecule originally developed as an anti-HIV drug
because of its ability to inhibit the CXC chemokine receptor 4 (CXCR4), a
co-receptor for HIV entry into CD4+ T lymphocytes . Early clinical
trials of plerixafor revealed a remarkable ability to increase CD34 cells in
peripheral blood. Plerixafor mobilizes CD34 cells by preventing chemokine
stromal cell-derived factor-1α (SDF-1α) from binding to CXCR4 and
directing the CD34 cells to “home” to the bone marrow. Plerixafor is
administered by subcutaneous injection after four days of G-CSF treatment and
11 hours prior to leukapheresis; it can be used with G-CSF for up to four
continuous days. Plerixafor is eliminated primarily by the renal route and must
be dose-adjusted for patients with renal impairment. The drug is
well-tolerated; the most common adverse effects associated with its use are
injection site reactions, GI disturbances, dizziness, fatigue, and headache.
Toxicity
Although the three
growth factors have similar effects on neutro-phil counts, G-CSF and
pegfilgrastim are used more frequently than GM-CSF because they are better
tolerated. G-CSF andpegfilgrastim can cause bone pain, which clears when the
drugs are discontinued. GM-CSF can cause more severe side effects,
par-ticularly at higher doses. These include fever, malaise, arthralgias,
myalgias, and a capillary leak syndrome characterized by periph-eral edema and
pleural or pericardial effusions. Allergic reactions may occur but are
infrequent. Splenic rupture is a rare but serious complication of the use of
G-CSF for PBSC.
Related Topics