Chapter: Basic & Clinical Pharmacology : Agents Used in Anemias; Hematopoietic Growth Factors
Megakaryocyte Growth Factors
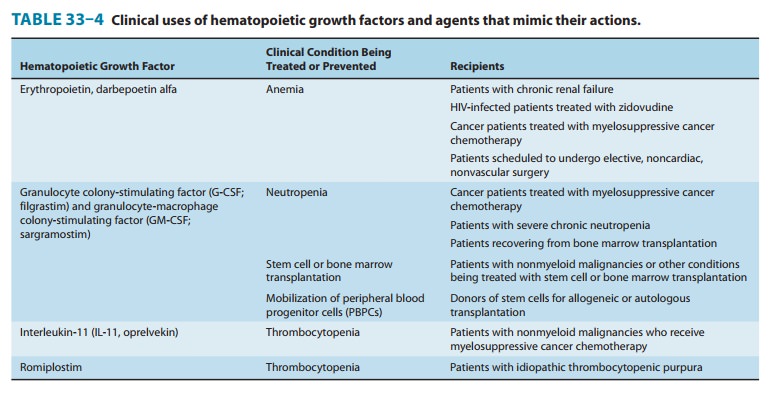
MEGAKARYOCYTE GROWTH FACTORS
Patients
with thrombocytopenia have a high risk of hemorrhage. Although platelet
transfusion is commonly used to treat throm-bocytopenia, this procedure can
cause adverse reactions in the recipient; furthermore, a significant number of
patients fail to exhibit the expected increase in platelet count. Thrombopoietin and IL-11 both appear to be key endogenous regulators of plate-let
production. A recombinant form of IL-11 was the first agent to gain FDA
approval for treatment of thrombocytopenia. Recombinant human thrombopoietin
and a pegylated form of a shortened human thrombopoietin protein underwent
extensive clinical investigation in the 1990s. However, further develop-ment
was abandoned after autoantibodies to the native throm-bopoietin formed in
healthy human subjects and caused thrombocytopenia. Efforts shifted to
investigation of novel, non-immunogenic peptide agonists of the thrombopoietin
receptor, which is known as Mpl. The first of these—romiplostim—was approved by the FDA for idiopathic thrombocytopenic
purpura in 2008.
Chemistry & Pharmacokinetics
Interleukin-11 is
a 65–85 kDa protein produced by fibroblastsand stromal cells in the bone
marrow. Oprelvekin, the recombi-nant
form of IL-11 approved for clinical use (Table 33–4), is produced by expression
in Escherichia coli. The half-life of
IL-11 is 7–8 hours when the drug is injected subcutaneously.
Romiplostim (AMG
531) is a member of new class of thera-peutics called “peptibodies,” which are
peptides with key biologic activities covalently linked to antibody fragments
that serve to extend the peptide’s half-life. Romiplostim contains two
disulfide-bonded human Fc fragments,
each covalently attached through a polyglycine sequence to a peptide chain
containing two Mpl-binding peptides that are linked to one another by a second
poly-glycine sequence. The Mpl-binding peptide was selected from a peptide
library based on its ability in cell assays to activate the thrombopoietin
receptor. The Mpl-binding peptide has no sequence homology with human
thrombopoietin and there is no evidence in animal or human studies that the
Mpl-binding pep-tide or romiplostim induces antibodies to thrombopoietin. After
subcutaneous administration, romiplostim is eliminated by the
reticuloendothelial system with an average half-life of 3–4 days. Its half-life
is inversely related to the serum platelet count; it has a longer half-life in
patients with thrombocytopenia and a shorter half-life in patients whose
platelet counts have recovered to nor-mal levels.Eltrombopag, an orally active small molecule agonist at thethrombopoietin
receptor, was licensed in 2008 for use in patients with severe idiopathic
thrombocytopenia who have failed to respond adequately to first-line
treatments. Because of concerns about hepatotoxicity and hemorrhage,
eltrombopag is restricted to use by registered physicians and patients and its
use requires close monitoring of liver enzymes.
Pharmacodynamics
Interleukin-11 acts
through a specific cell surface cytokine recep-tor to stimulate the growth of
multiple lymphoid and myeloid cells. It acts synergistically with other growth
factors to stimulate the growth of primitive megakaryocytic progenitors and,
most importantly, increases the number of peripheral platelets and neutrophils.
Romiplostim has high
affinity for the human Mpl receptor. It causes a dose-dependent increase in
platelet count that begins on day 5 after subcutaneous administration and peaks
at days 12–15.
Clinical Pharmacology
Interleukin-11 is
approved for the secondary prevention of throm-bocytopenia in patients
receiving cytotoxic chemotherapy for treatment of nonmyeloid cancers. Clinical
trials show that it reduces the number of platelet transfusions required by
patients who experience severe thrombocytopenia after a previous cycle of
chemotherapy. Although IL-11 has broad stimulatory effects on hematopoietic
cell lineages in vitro, it does not appear to have significant effects on the
leukopenia caused by myelosuppressive chemotherapy. Interleukin-11 is given by
subcutaneous injection at a dose of 50 mcg/kg/d. It is started 6–24 hours after
completionof chemotherapy and continued for 14–21 days or until the plate-let
count passes the nadir and rises to more than 50,000 cells/μL.
In patients with
chronic idiopathic thrombocytopenia (ITP) who failed to respond adequately to
previous treatment with ste-roids, immunoglobulins, or splenectomy, romiplostim
signifi-cantly increased platelet count in most patients. In a 6-week
placebo-controlled study in which patients were treated weekly with 1 or 3
mcg/kg, 12 of 16 patients reached the targeted platelet range of 50,000–450,000
platelets/μL.
Romiplostim does not appear to decrease the rate of platelet destruction in ITP
as platelet counts returned to pretreatment levels after the drug’s
discontinu-ation. An open label trial found that many patients maintained a
platelet count of 100,000 platelets/μL or higher over a 48-week period and that
over half of the patients were able to discontinue other therapies. Romiplostim
is initiated as a weekly subcutaneous dose of 1 mcg/kg and then continued at
the lowest dose required to maintain a platelet count of at least 50,000
platelets/μL.
Toxicity
The
most common adverse effects of IL-11 are fatigue, headache, dizziness, and
cardiovascular effects. The cardiovascular effects include anemia (due to
hemodilution), dyspnea (due to fluid accu-mulation in the lungs), and transient
atrial arrhythmias. Hypokalemia has also been seen in some patients. All of
these adverse effects appear to be reversible.
Romiplostim
appears to be well tolerated except for a mild head-ache on the day of
administration. A potential long-term concern is that two patients treated with
romiplostim had an increase in bone marrow reticulin, a possible marker of
myelodysplastic or myelopro-liferative processes. However, neither patient had
evidence of increased collagen fibrosis or of abnormal bone marrow
cytogenetics.
Related Topics