Chapter: Basic & Clinical Pharmacology : Agents Used in Anemias; Hematopoietic Growth Factors
Iron - Agents Used In Anemias
AGENTS USED IN ANEMIAS
IRON
Basic Pharmacology
Iron
deficiency is the most common cause of chronic anemia. Like other forms of
chronic anemia, iron deficiency anemia leads to pallor, fatigue, dizziness,
exertional dyspnea, and other gener-alized symptoms of tissue hypoxia. The
cardiovascular adaptations to chronic anemia—tachycardia, increased cardiac
output, vaso-dilation—can worsen the condition of patients with underlying
cardiovascular disease.Iron forms the nucleus of the iron-porphyrin heme ring,
which together with globin chains forms hemoglobin. Hemoglobin reversibly binds
oxygen and provides the critical mechanism for oxygen delivery from the lungs
to other tissues. In the absence of adequate iron, small erythrocytes with
insufficient hemoglobin are formed, giving rise to microcytic hypochromic anemia. Iron-containing heme is also an
essential component of myo-globin, cytochromes, and other proteins with diverse
biologic functions.
Pharmacokinetics
Free
inorganic iron is extremely toxic, but iron is required for essential proteins
such as hemoglobin; therefore, evolution has provided an elaborate system for
regulating iron absorption, trans-port, and storage (Figure 33–1). The system
uses specialized trans-port, storage, ferrireductase, and ferroxidase proteins
whose concentrations are controlled by the body’s demand for hemoglo-bin
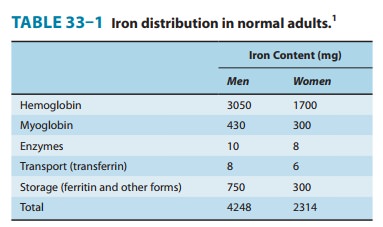
synthesis and adequate iron stores (Table 33–1). A peptide called hepcidin,
produced primarily by liver cells, serves as a key central regulator of the
system. Nearly all of the iron used to sup-port hematopoiesis is reclaimed from
catalysis of the hemoglobin in senescent or damaged erythrocytes. Normally,
only a small amount of iron is lost from the body each day, so dietary
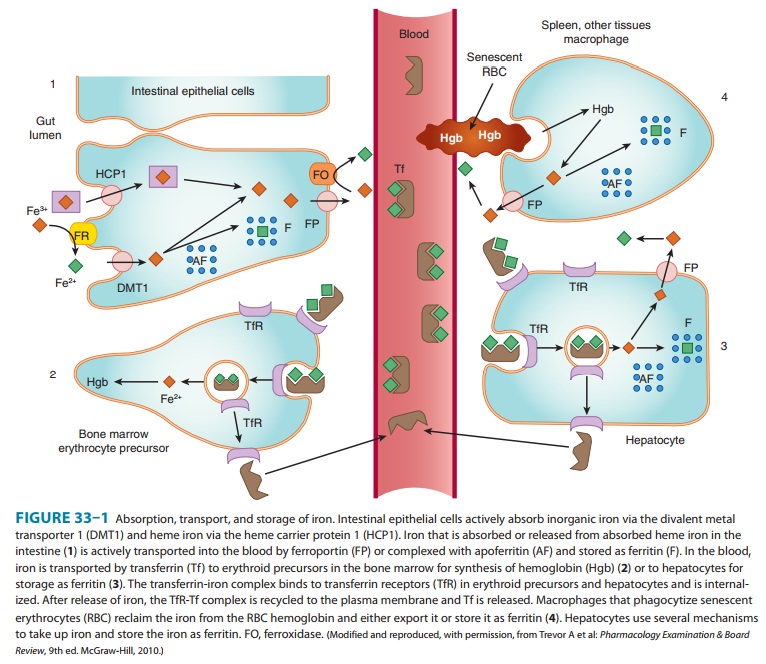
However, in special populations with either increased iron requirements (eg, growing children, pregnant women) or increased losses of iron (eg, menstruating women), iron requirements can exceed normal dietary supplies and iron deficiency can develop.
A. Absorption
The
average American diet contains 10–15 mg of elemental iron daily. A normal
individual absorbs 5–10% of this iron, or about 0.5–1 mg daily. Iron is
absorbed in the duodenum and proximal jejunum, although the more distal small
intestine can absorb iron if necessary. Iron absorption increases in response
to low iron stores or increased iron requirements. Total iron absorption
increases to 1–2 mg/d in menstruating women and may be as high as 3–4 mg/d in
pregnant women.Iron is available in a wide variety of foods but is especially
abundant in meat. The iron in meat protein can be efficiently absorbed, because
heme iron in meat hemoglobin and myoglobin can be absorbed intact without first
having to be dissociated into elemental iron (Figure 33–1). Iron in other
foods, especially veg-etables and grains, is often tightly bound to organic
compounds and is much less available for absorption. Nonheme iron in foods and
iron in inorganic iron salts and complexes must be reduced by a ferrireductase
to ferrous iron (Fe2+)
before it can be absorbed by intestinal mucosal cells.
Iron
crosses the luminal membrane of the intestinal mucosal cell by two mechanisms:
active transport of ferrous iron by the divalent metal transporter DMT1, and
absorption of iron com-plexed with heme (Figure 33–1). Together with iron split
from absorbed heme, the newly absorbed iron can be actively trans-ported into
the blood across the basolateral membrane by a trans-porter known as
ferroportin and oxidized to ferric iron (Fe3+)
by the ferroxidase hephaestin. The liver-derived hepcidin inhibits intestinal
cell iron release by binding to ferroportin and triggering its internalization
and destruction. Excess iron is stored in intesti-nal epithelial cells as
ferritin, a water-soluble complex consisting of a core of ferric hydroxide
covered by a shell of a specialized storage protein called apoferritin.
B. Transport
Iron is transported in
the plasma bound to transferrin, a β-globulin that can
bind two molecules of ferric iron (Figure 33–1). The transferrin-iron complex
enters maturing erythroid cells by a specific receptor mechanism. Transferrin
receptors—integral membrane glycoproteins present in large numbers on proliferating
erythroid cells—bind and internalize the transferrin-iron complex through the
process of receptor-mediated endocytosis. In endo-somes, the ferric iron is
released, reduced to ferrous iron, and transported by DMT1 into the cytoplasm,
where it is funneled into hemoglobin synthesis or stored as ferritin. The
transferrin-transferrin receptor complex is recycled to the cell membrane,
where the transferrin dissociates and returns to the plasma. This process
provides an efficient mechanism for supplying the iron required by developing
red blood cells.
Increased
erythropoiesis is associated with an increase in the number of transferrin
receptors on developing erythroid cells and a reduction in hepatic hepcidin
release. Iron store depletion and iron deficiency anemia are associated with an
increased concentra-tion of serum transferrin.
C. Storage
In addition to the
storage of iron in intestinal mucosal cells, iron is also stored, primarily as
ferritin, in macrophages in the liver, spleen, and bone, and in parenchymal
liver cells (Figure 33–1). The mobilization of iron from macrophages and
hepatocytes is primarily controlled by hepcidin regulation of ferroportin
activity. Low hepcidin concentrations result in iron release from these storage
sites; high hepcidin concentrations inhibit iron release. Ferritin is
detectable in serum. Since the ferritin present in serum is in equilibrium with
storage ferritin in reticuloendothelial tissues, the serum ferritin level can
be used to estimate total body iron stores.
D. Elimination
There is no mechanism
for excretion of iron. Small amounts are lost in the feces by exfoliation of
intestinal mucosal cells, and trace amounts are excreted in bile, urine, and
sweat. These losses account for no more than 1 mg of iron per day. Because the
body’s ability to excrete iron is so limited, regulation of iron balance must
be achieved by changing intestinal absorption and storage of iron, in response
to the body’s needs. As noted below, impaired regula-tion of iron absorption
leads to serious pathology.
Clinical Pharmacology
A. Indications for the Use of Iron
The
only clinical indication for the use of iron preparations is the treatment or
prevention of iron deficiency anemia. This manifests as a hypochromic,
microcytic anemia in which the erythrocyte mean cell volume (MCV) and the mean
cell hemoglobin concen-tration are low (Table 33–2). Iron deficiency is
commonly seen in populations with increased iron requirements. These include
infants, especially premature infants; children during rapid growth periods;
pregnant and lactating women; and patients with chronic kidney disease who lose
erythrocytes at a relatively high rate during hemodialysis and also form them
at a high rate as a result of treatment with the erythrocyte growth factor
erythropoietin . Inadequate iron absorption can also cause iron defi-ciency.
This is seen after gastrectomy and in patients with severe small bowel disease
that results in generalized malabsorption.
The most common cause of iron deficiency in adults is blood loss. Menstruating women lose about 30 mg of iron with each menstrual period; women with heavy menstrual bleeding may lose much more.
Thus, many
premenopausal women have low iron stores or even iron deficiency. In men and
postmenopausal women, the most common site of blood loss is the
gastrointestinal tract. Patients with unexplained iron deficiency anemia should
be evaluated for occult gastrointestinal bleeding.
B. Treatment
Iron deficiency anemia
is treated with oral or parenteral iron preparations. Oral iron corrects the
anemia just as rapidly and completely as parenteral iron in most cases if iron
absorption from the gastrointestinal tract is normal. An exception is the high
requirement for iron of patients with advanced chronic kidney disease who are
undergoing hemodialysis and treatment with erythropoietin; for these patients,
parenteral iron administration is preferred.
1. Oral iron therapy—A wide variety of oral iron prepara-tions is available. Because ferrous iron is most efficiently absorbed, ferrous salts should be used. Ferrous sulfate, ferrous gluconate, and ferrous fumarate are all effective and inexpensive and are recommended for the treatment of most patients.
Different
iron salts provide different amounts of elemental iron, as shown in Table 33–3.
In an iron-deficient individual, about 50–100 mg of iron can be incorporated
into hemoglobin daily, and about 25% of oral iron given as ferrous salt can be
absorbed. Therefore, 200–400 mg of elemental iron should be given daily to
correct iron deficiency most rapidly. Patients unable to tolerate such large
doses of iron can be given lower daily doses of iron, which results in slower
but still complete correction of iron deficiency. Treatment with oral iron should
be continued for 3–6 months after correction of the cause of the iron loss.
This corrects the anemia and replenishes iron stores.
Common adverse effects of oral iron therapy include nausea, epigastric discomfort, abdominal cramps, constipation, and diar-rhea. These effects are usually dose-related and can often be over-come by lowering the daily dose of iron or by taking the tablets immediately after or with meals. Some patients have less severe gastrointestinal adverse effects with one iron salt than another and benefit from changing preparations. Patients taking oral iron develop black stools; this has no clinical significance in itself but may obscure the diagnosis of continued gastrointestinal blood loss.
2. Parenteral iron therapy—Parenteral
therapy should bereserved for patients with documented iron deficiency who are
unable to tolerate or absorb oral iron and for patients with extensive chronic
anemia who cannot be maintained with oral iron alone. This includes patients
with advanced chronic renal disease requiring hemodialysis and treatment with
erythropoie-tin, various postgastrectomy conditions and previous small bowel
resection, inflammatory bowel disease involving the proximal small bowel, and
malabsorption syndromes.
The
challenge with parenteral iron therapy is that parenteral administration of
inorganic free ferric iron produces serious dose-dependent toxicity, which
severely limits the dose that can be administered. However, when the ferric
iron is formulated as a colloid containing particles with a core of iron
oxyhydroxide sur-rounded by a core of carbohydrate, bioactive iron is released
slowly from the stable colloid particles. In the United States, the three
available forms of parenteral iron are iron
dextran, sodium ferricgluconate complex, and iron sucrose.
Iron dextran is
a stable complex of ferric oxyhydroxide anddextran polymers containing 50 mg of
elemental iron per millili-ter of solution. It can be given by deep
intramuscular injection or by intravenous infusion, although the intravenous
route is used most commonly. Intravenous administration eliminates the local
pain and tissue staining that often occur with the intramuscular route and
allows delivery of the entire dose of iron necessary to correct the iron
deficiency at one time. Adverse effects of intrave-nous iron dextran therapy
include headache, light-headedness, fever, arthralgias, nausea and vomiting,
back pain, flushing, urti-caria, bronchospasm, and, rarely, anaphylaxis and death.
Owing to the risk of a hypersensitivity reaction, a small test dose of iron
dextran should always be given before full intramuscular or intra-venous doses
are given. Patients with a strong history of allergy and patients who have
previously received parenteral iron dextran are more likely to have
hypersensitivity reactions after treatment with parenteral iron dextran. The
iron dextran formulations used clinically are distinguishable as
high-molecular-weight and low-molecular-weight forms. In the United States, the
InFeD preparation is a low-molecular-weight form while DexFerrum is a
high-molecular-weight form. Clinical data—primarily from observational
studies—indicate that the risk of anaphylaxis is largely associated with
high-molecular-weight formulations.
Sodium ferric gluconate complex and iron-sucrose complex
are
alternative parenteral iron preparations. These agents can be given only by the
intravenous route. They appear to be less likely than high-molecular-weight
iron dextran to cause hypersensitivity reactions.
For
patients treated chronically with parenteral iron, it is important to monitor
iron storage levels to avoid the serious toxic-ity associated with iron
overload. Unlike oral iron therapy, which is subject to the regulatory
mechanism provided by the intestinal uptake system, parenteral
administration—which bypasses this regulatory system—can deliver more iron than
can be safely stored. Iron stores can be estimated on the basis of serum
concentrations of ferritin and the transferrin saturation, which is the ratio
of the total serum iron concentration to the total iron-binding capacity
(TIBC).
Clinical Toxicity
A. Acute Iron Toxicity
Acute iron toxicity is
seen almost exclusively in young children who accidentally ingest iron tablets.
As few as 10 tablets of any of the commonly available oral iron preparations
can be lethal in young children. Adult patients taking oral iron preparations
should be instructed to store tablets in child-proof containers out of the
reach of children. Children who are poisoned with oral iron experience
necrotizing gastroenteritis, with vomiting, abdominal pain, and bloody diarrhea
followed by shock, lethargy, and dysp-nea. Subsequently, improvement is often
noted, but this may be followed by severe metabolic acidosis, coma, and death.
Urgent treatment is necessary. Whole
bowel irrigation should be performed
to flush out unabsorbed pills. Deferoxamine,
a potent iron-chelating compound, can be given intravenously to bind iron that
has already been absorbed and to promote its excre-tion in urine and feces.
Activated charcoal, a highly effective adsorbent for most toxins, does not
bind iron and thus is ineffec-tive. Appropriate supportive therapy for
gastrointestinal bleeding, metabolic acidosis, and shock must also be provided.
B. Chronic Iron Toxicity
Chronic iron toxicity
(iron overload), also known as hemochro-matosis,
results when excess iron is deposited in the heart, liver,pancreas, and
other organs. It can lead to organ failure and death. It most commonly occurs
in patients with inherited hemochroma-tosis, a disorder characterized by
excessive iron absorption, and in patients who receive many red cell
transfusions over a long period of time (eg, individuals with β-thalassemia).
Chronic iron overload
in the absence of anemia is most effi-ciently treated by intermittent
phlebotomy. One unit of blood can be removed every week or so until all of the
excess iron is removed. Iron chelation therapy using parenteral deferoxamine or the oral iron chelator deferasirox is less efficient as well as more
complicated, expensive, and hazardous, but it may be the only option for iron
overload that cannot be managed by phle-botomy, as is the case for many
individuals with inherited and acquired causes of refractory anemia such as
thalassemia major, sickle cell anemia, aplastic anemia, etc.
Related Topics