Chapter: Medical Surgical Nursing: Management of Patients With Neurologic Infections, Autoimmune Disorders, and Neuropathies
Myasthenia Gravis - Autoimmune Processes
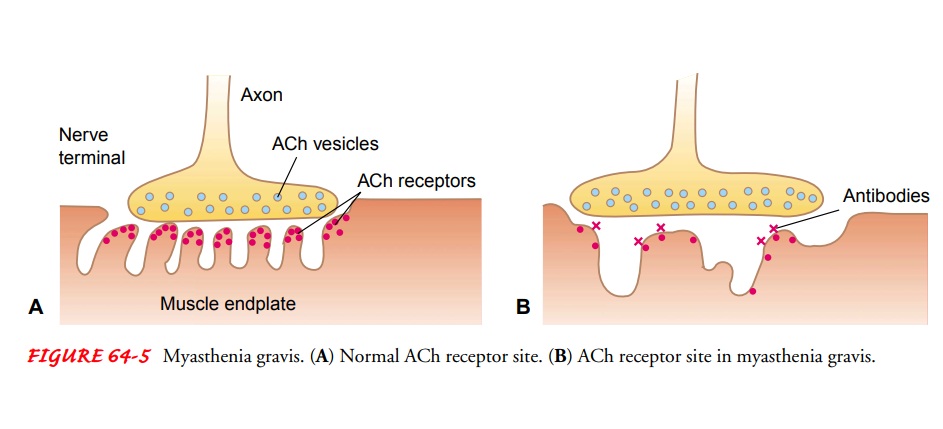
MYASTHENIA GRAVIS
Myasthenia gravis, an autoimmune disorder affecting the
myo-neural junction, is characterized by varying degrees of weakness of the
voluntary muscles. Women tend to develop the disease at an earlier age (20 to
40 years of age) compared to men (60 to 70 years of age), and women are
affected more frequently (Heitmiller, 1999).
Pathophysiology
Normally, a chemical impulse precipitates the release of
acetyl-choline from vesicles on the nerve terminal at the myoneural junction.
The acetylcholine attaches to receptor sites on the motor end plate,
stimulating muscle contraction. Continuous binding of acetylcholine to the
receptor site is required for muscular con-traction to be sustained.
In myasthenia gravis,
autoantibodies directed at the acetyl-choline receptor sites impair
transmission of impulses across the myoneural junction. Therefore, fewer
receptors are available for stimulation, resulting in voluntary muscle weakness
that escalates with continued activity (Fig. 64-5). These antibodies are found
in 80% to 90% of the people with myasthenia gravis. Eighty per-cent of persons
with myasthenia gravis have either thymic hyper-plasia or a thymic tumor (Roos,
1999), and the thymus gland is believed to be the site of antibody production.
In patients who are antibody negative, it is believed that the offending
antibody is directed at a portion of the receptor site rather than the whole
complex.
Clinical Manifestations
The initial
manifestation of myasthenia gravis usually involves the ocular muscles.
Diplopia (double vision) and ptosis (drooping of the eyelids) are common.
However, the majority of patients also experience weakness of the muscles of
the face and throat (bulbar symptoms) and generalized weakness. Weakness of the
facial muscles will result in a bland facial expression. Laryngeal involvement
produces dysphonia (voice
impairment) and in-creases the patient’s risk for choking and aspiration.
Generalized weakness affects all the extremities and the intercostal muscles,
resulting in decreasing vital capacity and respiratory failure. Myasthenia
gravis is purely a motor disorder with no effect on sensation or coordination.
Assessment and Diagnostic Findings
An anticholinesterase
test is used to diagnose myasthenia gravis. Anticholinesterase agents stop the
breakdown of acetylcholine, thereby increasing acetylcholine availability.
Edrophonium chlo-ride (Tensilon) is injected intravenously, 2 mg at a time to a
total of 10 mg. Thirty seconds after injection, facial muscle weakness and
ptosis should resolve for about 5 minutes. This immediate improvement in muscle
strength after administration of this agent represents a positive test and
usually confirms the diagnosis. Atropine 0.4 mg should be available to control
the side effects of edrophonium, which include bradycardia, sweating, and
cramping (Roos, 1999).
The acetylcholine receptor antibody titers are elevated as in-dicated previously. Repetitive nerve stimulation tests record the electrical activity in targeted muscles after nerve stimulation. A 15% decrease in successive action potentials is observed in pa-tients with myasthenia gravis (Heitmiller, 1999). The thymus gland, which is a site of acetylcholine receptor antibody produc-tion, is enlarged in myasthenia gravis. MRI demonstrates this en-largement in 90% of cases (Wilkins & Bulkley, 1999).
Medical Management
Management of myasthenia
gravis is directed at improving function and reducing and removing circulating
antibodies. Therapeutic modalities include administration of anticholinesterase
agents and immunosuppressive therapy, plasmapheresis, and thymectomy.
PHARMACOLOGIC THERAPY
Anticholinesterase
agents such as pyridostigmine bromide (Mesti-non) and neostigmine bromide
(Prostigmin) provide symptomatic relief by increasing the relative
concentration of available acetyl-choline at the neuromuscular junction. Dosage
is increased grad-ually until maximal benefits (improved strength, less
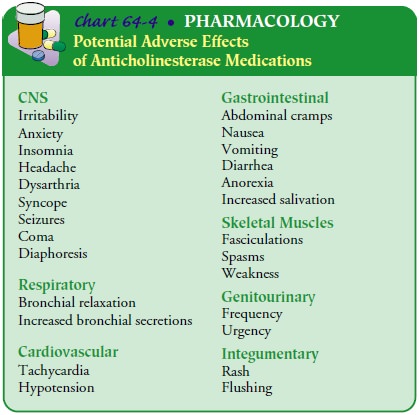
fatigue) are obtained. Adverse effects of anticholinesterase therapy include
ab-dominal pain, diarrhea, nausea, and increased oropharyngeal secre-tions.
Pyridostigmine tends to have fewer side effects (Chart 64-4). Improvement with
anticholinesterase therapy is not complete or long-lasting (Heitmiller, 1999).
The goal of immunosuppressive therapy is to reduce the pro-duction of the antibody. Corticosteroids suppress the patient’s immune response, thus decreasing the amount of antibody pro-duction. As the corticosteroid dosage is gradually increased, the anticholinesterase dosage is lowered. The patient’s ability to main-tain effective respirations and to swallow is monitored through-out. Prednisone, taken on alternate days to lower the incidence of side effects, appears to be successful in suppressing the disease. The patient sometimes shows a marked decrease in muscle strength right after therapy is started, but this is usually only temporary Cytotoxic medications have also been used, although the precise mechanism of action in myasthenia is not fully under-stood.
Medications
such as azathioprine (Imuran), cyclophos-phamide (Cytoxan), and cyclosporine
reduce the circulating anti-acetylcholine receptor antibody titers. Side effects
are significant; therefore, these agents are reserved for patients who do not
re-spond to other forms of therapy.
A number of medications
are contraindicated for patients with myasthenia gravis because they worsen
myasthenic symptoms. Risks and benefits should be weighed by the physician and
the patient before taking any new medications, including antibiotics,
cardiovascular medications, antiseizure and psychotropic med-ications,
morphine, quinine and related agents, beta-blockers, and nonprescription
medications. Procaine (Novocain) should be avoided, and the patient’s dentist
is so advised.
PLASMAPHERESIS
Plasma exchange
(plasmapheresis) is a technique used to treat exacerbations. The patient’s
plasma and plasma components are removed through a centrally placed large-bore
double-lumen catheter. The blood cells and antibody-containing plasma are
sep-arated; then the cells and a plasma substitute are reinfused. Plasma
exchange produces a temporary reduction in the titer of circulat-ing
antibodies. Plasma exchange improves the symptoms in 75% of patients, although
improvement lasts only a few weeks unless plasmapheresis is continued or other
forms of treatment such as immunosuppression with corticosteroids are initiated
(Bedlack & Sanders, 2000). IV immune globulin (IVIG) has recently been
shown to be nearly as effective as plasmapheresis in controlling symptom
exacerbation (Qureshi, Choudhry, Akbar et al., 1999). However, neither therapy
is a cure as it does not stop the pro-duction of the acetylcholine receptor
antibodies.
SURGICAL MANAGEMENT
Thymectomy (surgical removal of the thymus gland) can
produce antigen-specific immunosuppression and result in clinical im-provement.
It can decrease or eliminate the need for medication. In one study 92% of post-thymectomy
patients had symptomatic improvement, with 50% of them no longer requiring
pharmaco-logic therapy (Wilkins & Bulkley, 1999). The entire gland must be
removed for optimal clinical outcomes; therefore, surgeons prefer the
transsternal surgical approach. After surgery, the pa-tient is monitored in an
intensive care unit, with special atten-tion to respiratory function. After the
thymus gland is removed, it may take up to 1 year for the patient to benefit
from the pro-cedure due to the long life of circulating T cells (Wilkins &
Bulkley, 1999).
Complications: Myasthenic Crisis Versus Cholinergic Crisis
A myasthenic crisis is
an exacerbation of the disease process char-acterized by severe generalized
muscle weakness and respiratory and bulbar weakness that may result in
respiratory failure. Crisis may result from disease exacerbation or a specific
precipitating event. The most common precipitator is infection; others include
medication change, surgery, pregnancy, and high environmental temperature (Bella
& Chad, 1998).
Symptoms of
anticholinergic overmedication (cholinergic crisis) may mimic the symptoms of
exacerbation. Differentiation can be achieved with the edrophonium chloride
(Tensilon) test. The patient with myasthenic crisis improves immediately
fol-lowing administration of edrophonium, while the patient with cholinergic
crisis may experience no improvement or deteriorate. If myasthenic crisis is
diagnosed, neostigmine methylsulfate (PMS-Neostigmine, Prostigmin) is
administered intramuscularly or intravenously until the patient is able to
swallow oral anti-cholinesterase medications. Plasmapheresis and IVIG, which
re-duce the antibody load, also may be used to treat myasthenic crisis. If
cholinergic crisis is identified, all anticholinesterase med-ications are
stopped. The patient receives atropine (Atropine sul-fate), the antidote for
the anticholinesterase medications.
Neuromuscular
respiratory failure is the critical complication of crisis. Respiratory muscle
and bulbar weakness combine to cause respiratory compromise. Weak respiratory
muscles will not sup-port inhalation. An inadequate cough and an impaired gag
reflex caused by bulbar weakness result in poor airway clearance. Values on two
respiratory function tests, the negative inspiratory force and vital capacity,
will be the first clinical signs to deteriorate. Careful monitoring of these
values enables the nurse to monitor for impending respiratory failure.
Respiratory support and airway protection are key interventions for the nurse caring
for the pa-tient in crisis. Endotracheal intubation and mechanical ventila-tion
may be needed. Nutritional support may be needed if the patient is intubated
for a long period.
Nursing Management
Because myasthenia gravis is a chronic disease and most
patients are seen on an outpatient basis, much of the nursing care focuses on
patient and family teaching. Educational topics for outpatient self-care
include medication management, energy conservation, strategies to help with
ocular manifestations, and prevention and management of complications.
Medication management is a crucial component of ongoing
care. Understanding the action of the medications and taking them on schedule
is emphasized, as are the consequences of de-laying medication and the signs and
symptoms of myasthenic and cholinergic crisis. The patient can determine the
best times for daily dosing by keeping a diary to determine fluctuation of
symp-toms and to learn when the medication is wearing off. The med-ication
schedule can then be manipulated to maximize strength throughout the day.
The patient is also taught srategies to conserve energy.
To do this, the nurse helps the patient identify the best times for rest
pe-riods throughout the day. If the patient lives in a two-story home, the nurse
can suggest that frequently used items such as hygiene products, cleaning
products, and snacks be kept on each floor to minimize travel between floors.
The patient is encouraged to apply for a handicapped license plate to minimize
walking from parking spaces and to schedule activities to coincide with peak
en-ergy and strength levels.
To minimize the risk of
aspiration, mealtimes should coincide with the peak effects of
anticholinesterase medication. In addition, rest before meals is encouraged to
reduce muscle fatigue. The pa-tient is advised to sit upright during meals with
the neck slightly flexed to facilitate swallowing. Soft foods in gravy or
sauces can be swallowed more easily; if choking occurs frequently, the nurse
can suggest pureéing food to a pudding consistency. Suction should be available
at home and the patient and family instructed in its use. Gastrostomy feedings
may be necessary in some patients to ensure adequate nutrition.
Impaired vision results
from ptosis of one or both eyelids, decreased eye movement, or double vision.
To prevent corneal damage when the eyelids do not close completely, the patient
is instructed to tape the eyes closed for short intervals and regularlyinstill
artificial tears. Patients who wear eyeglasses can have “crutches” attached to
help lift the eyelids. Patching one eye can help with double vision.
The patient is reminded
of the importance of maintaining health promotion practices and of following
health care screen-ing recommendations. Factors that will exacerbate symptoms
and potentially cause crisis should be noted and avoided: emotional stress,
infections (particularly respiratory infections), vigorous physical activity,
some medications, and high environmental temperature. The Myasthenia Gravis
Foundation of America provides support groups, services, and educational
materials for patients, families, and health care providers.
MANAGING MYASTHENIC AND CHOLINERGIC CRISES
Respiratory
distress and varying degrees of dysphagia (difficulty swallowing), dysarthria
(difficulty speaking), eyelid ptosis, diplopia, and prominent muscle weakness
are symptoms of myasthenic and cholinergic crisis. The patient is placed in an
intensive care unit for constant monitoring because of associated intense and
sudden fluctuations in clinical condition.
IV edrophonium chloride
(Tensilon) is used to differentiate the type of crisis. It improves the
condition of the patient in myas-thenic crisis and temporarily worsens that of
the patient in cholin-ergic crisis. If the patient is in true myasthenic
crisis, neostigmine methylsulfate is administered intramuscularly or
intravenously. If the edrophonium test is inconclusive or there is increasing
respi-ratory weakness, all anticholinesterase medications are stopped, and
atropine sulfate is given to reduce excessive secretions.
Providing ventilatory assistance takes precedence in the
im-mediate management of the patient with myasthenic crisis. On-going
assessment for respiratory failure is essential. The nurse assesses the
respiratory rate, depth, and breath sounds and mon-itors pulmonary function
parameters (vital capacity and negative inspiratory force) to detect pulmonary
problems before respira-tory dysfunction progresses. Blood is drawn for
arterial blood gas analysis. Endotracheal intubation and mechanical ventilation
may be needed.
When there is severe weakness of the abdominal,
intercostal, and pharyngeal muscles, the patient cannot cough, take deep
breaths, or clear secretions. Chest physical therapy, including postural
drainage to mobilize secretions, and suctioning to re-move secretions may have
to be performed frequently. (Postural drainage should not be performed for 30
minutes after feeding.)
Assessment strategies and supportive measures include the
following:
·
Arterial blood gases, serum
electrolytes, input and output, and daily weight are monitored.
·
If the patient cannot swallow,
nasogastric tube feedings may be prescribed.
·
Sedatives and tranquilizers
are avoided because they aggra-vate hypoxia and hypercapnia and can cause
respiratory and cardiac depression.
Related Topics