Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Monoclonal Antibodies in Cancer
Monoclonal Antibodies in Cancer
INTRODUCTION
Cancer is the second-leading cause of death in the United States with
one of every four deaths attributable to cancer (Jemal et al., 2006). However,
when mortality rates were considered on the basis of age, cancer began to
surpass heart disease as the leading cause of death for persons younger than 85
years starting in 1999 (Jemal et al., 2006). Survival rates have improved only
modestly over the last several decades (e.g., 5 year relative survival rates
from 1995 to 2001 were 65% compared to 50% from 1974 to 1976) with most
survival advances occurring through earlier detection of cancer rather than
through treatment advances (American Cancer Society, 2006). New treatments that
can exploit intrinsic differencesbetween normal and neoplastic cells are needed
to offer patients additional options besides traditional treatment modalities
of surgery, radiation, and che-motherapy (Zangmeister-Wittke, 2005).
Therapeutic monoclonal antibodies represent a new type of treatment that can be
used to improve overall survival, increase the time to progression, and delay
the time to recurrence of many oncologic diseases. These drugs will provide
many treatment alternatives for cancer patients.
Because of the high incidence, morbidity, and mortality rates associated
with cancer, staying abreast of emerging therapeutic innovations in cancer care
are paramount for health care professionals and scientists, both professionally
and personally. This chapter summarizes pertinent points about the currently
approved oncologic monoclonal antibodies in the United States. Antibodies are
organized based on their target [i.e., CD cell, epidermal growth factor
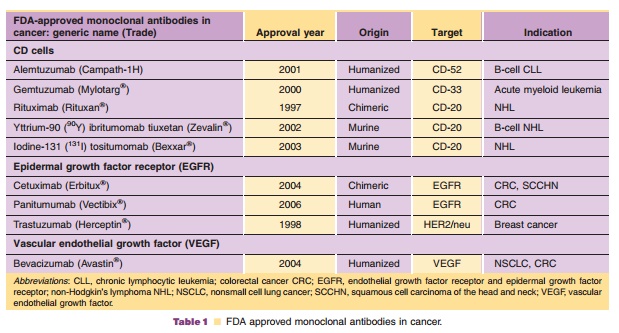
receptor (EGFR), and vascular endothelial growth factor (VEGF) receptor]. Table
1 summarizes the current FDA approved monoclonal antibodies for cancer
indications, year of approval, their origin and target, and appropriate
indications that will be discussed (Adams and Weiner, 2005).
Related Topics