Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Monoclonal Antibodies in Cancer
Trastuzumab - Classes of Monoclonal Antibodies: Endothelial Growth Factor Receptor (EGFR) Inhibitors
CLASSES OF MONOCLONAL ANTIBODIES: ENDOTHELIAL GROWTH FACTOR RECEPTOR (EGFR) INHIBITORS
EGFR or HER-1 and EGFR 2 or HER-2 are transmembrane glycoproteins constitutively expressed in many normal epithelial tissues including skin and hair follicle and are overexpressed in many cancers including the colon, rectum and breast. EGFR and HER-2 are members of the Erb family of receptors that play a role in normal cell growth and differentiation. HER-2 protein overexpression can occur in up to 30% of patients with metastatic breast cancer and is often associated with more aggressive disease, a fasterrelapse time, and an overall poor prognosis (Folkman, 1971; Miller, 2002).
Trastuzumab
Pharmacology and Mechanism of Action
Trastuzumab (Herceptin ) is a recombinant DNA-derived humanized MAb (IgG1 kappa) that selectively binds to the extracellular domain of the human HER-2 receptor with high affinity. This receptor-antibody interaction, through a series of other cellular actions, induces autophosphorylation of the tyrosine kinase internal domain resulting in decreased tumorigenic potential and possibly reversal of chemoresistance as shown in Figure 5.
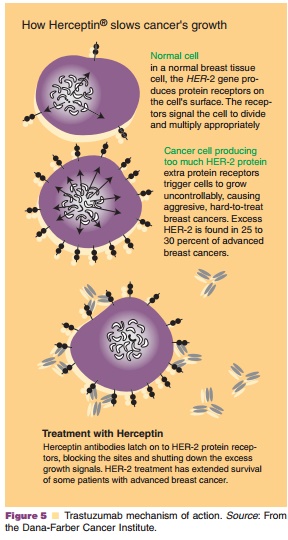
Pharmacokinetics/Pharmacodynamics
Weekly administration of trastuzumab exhibits dose-dependent pharmacokinetics. When 10 and 500 mg doses (administered by short duration intravenous infusions) were studied in women with metastatic breast cancer the mean half-life increased and clearance decreased with the increased dose. The observed average half-life was 1.7 days for the 10 mg dose and 12 days for the 500 mg dose. However, in studies analyzing the commonly used regimen for trastuzumab of an initial loading dose of 4 mg/kg followed by a 2 mg/kg weekly maintenance dose, the mean half-life was 5.8 days. Studies also suggest that age and serum creatinine do not effect the disposition of trastuzumab. It is also important to note that when trastuzumab is administered in combination with paclitaxel, a 1.5-fold elevation in serum concentrations of trastuzumab is observed as compared to when trastuzumab is administered in combination with anthracycline and cyclophosphamide (AC).
Indications and Clinical Efficacy
In both in vitro and in vivo studies trastuzumab was shown to inhibit the proliferation of human tumor cells that overexpress HER-2 (Hudziak et al., 1989; Baselga et al., 1998). Trastuzumab was approved by the FDA in September 1998 as a single agent for the treatment of patients having HER-2 overexpressing metastatic breast cancer and who had previously been treated with chemotherapy; in combination with paclitaxel in patients with HER-2 overexpressing metastatic breast cancer and had not received any prior chemotherapy (Genentech, 2006b). Trastuzumab has been found to have a 15% response rate in breast tumors overexpressing HER-2. However, this re-sponse rate increases to 25% when it is used in combination with chemotherapy.
The safety and efficacy of trastuzumab was determined in a multicenter, randomized, controlled clinical trial (H0648g) of 469 patients with HER2 overexpressing metastatic breast cancer who had not been treated for their metastatic disease (Slamon et al., 2001). Patients were randomized to receive either chemotherapy alone or chemotherapy plus trastuzu-mab. There were two chemotherapy subgroups. The first subgroup included patients who had previously been treated with an anthracycline in an adjuvant setting and thus received paclitaxel as chemotherapy in this study. The second subgroup included patients who had not received an anthracycline previously and received doxorubicin or epirubicin plus AC. Patients in the chemotherapy plus trastuzumab study arm had a significantly higher response rate, longer time to disease progression, a longer median duration of response, and a higher 1-year survival rate. Although
these results were observed in both chemotherapy subpopulations, the patients receiving paclitaxel plus trastuzumab had a greater effect than did patients receiving AC plus trastuzumab. In addition, a higher incidence of cardiac events occurred in the trastuzu-mab plus AC treatment group.
Another pivotal clinical trial (M77001) studied the combination of trastuzumab plus weekly or thrice weekly docetaxel in 188 patients who had no not been previously treated for their metastatic disease. After 24 months, a median overall survival of 31.2 months was observed for patients treated with trastuzumab plus docetaxel versus 22.7 months for patients treated with docetaxel alone (Marty et al., 2005). The outcome of these two trials has proven that trastuzu-mab plus a taxane is superior to a taxane alone and thus trastuzumab plus a taxane is now considered a standard of care for HER-2 positive metastatic breast cancer.
A multicenter, open-label, single arm clinical trial was conducted to assess the utility of trastuzu-mab as a single agent. This study enrolled 222 patients who had previously received one or more chemother-apy regimens for metastatic disease. Patients were treated with a trastuzumab loading dose of 4 mg/kg IV followed by 2 mg/kg IV weekly. A 14% response rate was observed (12% PR and 2% CR) in this study. As in the previous study, the degree of HER2 protein overexpression was a predictor of treatment response (Cobleigh et al., 1999). Preliminary results from other clinical trials looking at trastuzumab in combination with other chemotherapeutic agents include: trastu-zumab plus vinorelbine shows data supporting a high response rate when used as first line therapy (84%) (Burstein et al., 2001); in combination with gemcitabine a response rate of 38% was observed (O’Shaughnessy et al., 2004); and in combination with capecitabine a response rate of up to 60% was observed (Schaller et al., 2005). All of these studies suggest that trastuzumab is as tolerable as in the pivotal trials.
Safety Concerns
Trastuzumab has a black box warning for cardiomyo-pathy because of its potential to cause ventricular dysfunction and congestive heart failure. The severity and occurrence of cardiomyopathy was higher in patients who received anthracyclines and AC in combination with trastuzumab. Patients who require trastuzumab therapy must receive a full cardiac workup prior to the initiation of therapy and left ventricular function must be monitored during treat-ment. The most common adverse reaction is infusion reactions (usually mild to moderate), but rarely require discontinuation of therapy (Genentech,2006b). Other adverse effects associated with trastu-zumab are anemia and leukopenia, nausea/vomiting, diarrhea, and upper respiratory infections.
Pharmaceutical Considerations: Formulation, Other Routes of Administration, Dosing Regimens
Trastuzumab is only available for IV administration. It is commercially available as a white to pale yellow, preservative-free, lyophilized powder containing 440 mg of trastuzumab packaged sterilely in a vial. The powder can be reconstituted with 20 mL of the supplied bacteriostatic water for injection. The re-commended adult dosage is an initial loading dose of 4 mg/kg infused over 90 min followed by a weekly dose of 2 mg/kg infused over 90 min.
Related Topics