Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Monoclonal Antibodies in Cancer
Cetuximab - Classes of Monoclonal Antibodies: Endothelial Growth Factor Receptor (EGFR) Inhibitors
Cetuximab
Pharmacology and Mechanism of Action
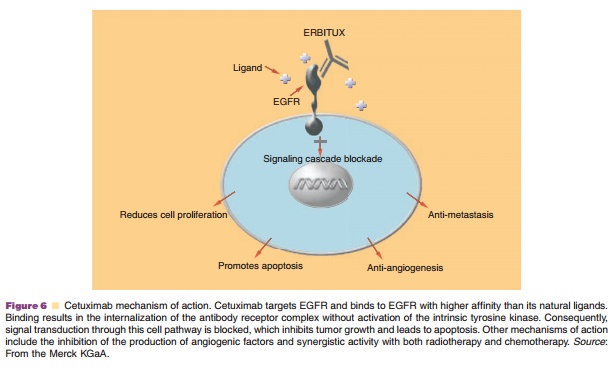
EGFR is an important marker in CRC because it is overexpressed in as much as 70% of CRC tumors and overexpression has been associated with decreased survival (Salomon et al., 1995). Cell signaling invol-ving EGFR (or HER-2 as with trastuzumab) is associated with neoplastic cell proliferation, angio-genesis, and resistance to apoptosis, among other tumorigenic characteristics as shown in Figure 6 (Castillo et al., 2004). Typically, advanced CRC is treated with fluoropyrimidine, irinotecan, andoxaliplatin, which have been shown to increase overall survival. However, once these drugs have been tried and are no longer effective, other treatment modalities including cetuximab have been found that can significantly prolong survival.
Pharmacokinetics/Pharmacodynamics
Dose escalation studies have shown that cetuximab exhibits nonlinear pharmacokinetics. The area under the concentration time curve shows a much greater proportionate increase than would be expected when the dose was increased from 20 to 400 mg/m2. In addition, the drug clearance decreased from 0.08 to 0.02 L/h/m2 with 20 and 200 mg/m2 doses. When doses greater than 200 mg/m2 were analyzed the clearance appeared to plateau. It was also found that when males and females were compared, female patients had a 25% lower intrinsic cetuximab clear-ance. Steady-state levels were reached by week 3 of cetuximab infusions with a 114 hr mean half-life when the recommended regimen of 400 mg/m2 (loading dose) followed by weekly 250 mg/m2 was adminis-tered (Nolting et al., 2006).
Indications and Clinical Efficacy
Cetuximab (Erbitux ) was approved by the FDA in February 2004 for chemoresistant CRC. However, several studies are ongoing to study the efficacy of cetuximab plus irinotecan or cetuximab plus an oxaliplatin-based chemotherapy regimen as first-line therapy. Cetuximab is a chimeric IgG1 MAb composed of the Fv region of a murine anti-EGFR antibody with human IgG heavy and kappa light chain constant regions (Bristol Myers Squibb, 2006). This antibody binds specifically to the extracellular domain of the human EGFR with a high affinity resulting in inhibition of cell growth, induction of apoptosis, decreased matrix metalloproteinase, and VEGF pro-duction. Cetuximab is currently indicated for use in combination with irinotecan for EGFR expressing, metastatic CRC in patients refractory to irinotecan-based chemotherapy; and as a single agent in patients intolerable to irinotecan-based chemotherapy (Bristol Myers Squibb, 2006). As with trastuzumab, the clinical study design was based on the assumption that EGFR protein expression in the tumor of interest is required in order to observe a tumor response from cetuximab therapy. However, recent studies suggest there is no correlation between EGFR expression and tumor response to cetuximab therapy (Cunningham et al., 2004).
In the pivotal BOND trial, 329 patients with metastatic EGFR expressing chemo-refractory CRC were randomized to receive either cetuximab plus irinotecan or cetuximab alone (Cunningham et al., 2004). Both study arms received a 400 mg/m2 loading dose followed by 250 mg/m2 weekly until either the patient had intolerable toxicities or disease progression occurred. The OR rate in the two treatment arms was 22.9% in patients receiving combination therapy (irinotecan plus cetuximab) and 10.8% in the cetuximab monotherapy group. In addition, two prespecified subpopulations were analyzed; an irinotecan-oxaliplatin failure group (irinotecan refractory patients who had previously been treated with and failed an oxaliplatin contain-ing regimen) and the irinotecan refractory group. The irinotecan-oxaliplatin failure subpopulation had a 23.8% response rate and a 2.9 month median time to disease progression for the cetuximab plus irinotecan study arm; and an 11.4% response rate and 1.5 month time to progression for the cetuximab monotherapy study arm. The irinotecan refractory subpopulation had a 25.8% and 14.5% response rate with a 4 month and 1.5 month time to progression for the cetuximab plus irinotecan and cetuximab monotherapy treatment groups, respectively. These data suggest that cetuximab plus chemotherapy will result in a higher tumor response rate than when cetuximab is given alone via overcoming irinotecan tumor resistance. There was no observed correlation between the level of the EGFR expression and response rate. Therefore, other genomic markers are needed to better define the correlation between EGFR inhibitors and tumor response rate.
A phase II study was conducted to assess the safety and efficacy of two monoclonal antibodies, cetuximab plus bevacizumab with or without irinote-can in irinotecan refractory CRC patients (Saltz et al., 2005). The cetuximab plus bevacizumab plus irinote-can resulted in a 37% response rate and 7.9 month time to progression as compared to 20% response rate and 5.6 month time to progression in the group that did not receive irinotecan. In addition, several small studies are assessing the utility of cetuximab as first line therapy in combination with other commonly used chemotherapy regimens (Hohler et al., 2004; Van Cutsem et al., 2004). The results of these studies seem very promising with a response rate ranging from 24% (patients receiving cetuximab and 5-fluorouracil/ leucovorin/oxaliplatin, FUFOX) up to 81% (patients receiving cetuximab and an oxaliplatin-containing regimen).
Safety Concerns
Cetuximab has a black box warning for severe infusion reactions occurring in approximately 3% of patients most of which (90%) was associated with the first infusion (Segaert and Van Cutsem, 2005). The types of infusion reactions observed include rapid onset of airway obstruction, urticaria, and hypoten-sion. Premedication with an H1-antagonist is required to help minimize the degree of the hypersensitivity reactions. Other serious side effects associated with cetuximab include severe diarrhea (when given in combination with irinotecan), dehydration, fever, interstitial lung disease, dermatologic toxicities, kid-ney failure, and pulmonary embolus. One of the most common side effects of cetuximab is acneiform eruptions (a maculopapular rash occurring in > 50% of patients), which are characteristic of EGFR block-ade and is a result of the role of EGFR in the maintenance of skin integrity. Furthermore, a positive correlation has been made with tumor response to cetuximab and severity of the skin rash (Cunnigham et al., 2004; Segaert and Van Cutsem, 2005). Other common side effects include xerosis, diarrhea, nausea, abdominal pain, vomiting, and constipation. Cardiopulmonary arrest, mouth sores, severe radia-tion skin reactions, weight loss, dry mouth, and difficulty swallowing may occur when cetuximab is given in combination with radiation therapy.
Pharmaceutical Considerations: Formulation, Other Routes of Administration, Dosing Regimens
Cetuximab is only available for IV administration. It is commercially available in a 50 mL vial containing 100 mg of cetuximab (2 mg/mL) formulated in a sterile, preservative free, colorless, clear liquid. The recommended dose (in combination therapy or as monotherapy) is 400 mg/m2 as a one time loadingdose infused intravenously over 120 min followed by a weekly maintenance dose of 250 mg/m2 infused over 60 min. Premedication with an H1 antagonist is recommended to circumvent infusion reactions. Patients who experience grade 1 or 2 infusion reactions require a decrease in the rate of infusion by 50%. Individuals who experience a grade 3 or 4 infusion reaction require permanent discontinuation of cetuximab therapy.
Related Topics