Chapter: Plant Anatomy:An Applied Approach: The leaf
Mesophyll - The leaf
The mesophyll
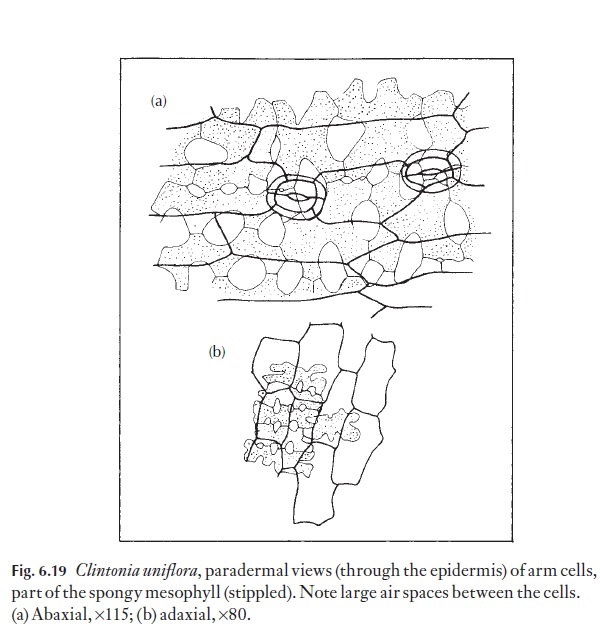
The mesophyll usually consists of the thin-walled parenchymatous cells containing chloroplasts, called chlorenchyma, and other thin-walled cells concerned with water, food or ergastic or so-called ‘waste product’ (e.g. crystals, tannins) storage. Leaves of dicotyledonous plants differ greatly from those of monocotyledonous plants and from those of gymnosperms and ferns. Of course, there is some degree of intergradation, but generally, it is possible to separate these leaves, using some basic diagnostic criteria. Dicotyledons generally have a mesophyll which is composed of two differ-ing photosynthetic cell types – palisade and spongy mesophyll cells; paren-chyma cells may be present between these. Some monocotyledons are also like this, but there is a wide range of cell forms in the chlorenchyma, and frequently palisade cells are not present. The classical division of mesophyll into palisade-like cells and spongy cells may be misleading in its oversim-plification. There are many intergrading cell shapes between the extremes. Figure 6.19 shows paradermal views of arm cells, part of the spongy tissue in Clintonia. Because some leaves lack a distinction of layers and others have very well marked layers, the mesophyll can be used as an aid to identifica-tion. It cannot often be used as a guide to the taxonomic position of a plant, but within a group of related plants there may be close similarities of ar-rangement. Environmental variations will not alter arrangements that are rigidly controlled by the genome. For example, palisade cells can be present next to the upper or to the lower surface, or to both. There are, however, striking changes that can occur to the layers themselves. In some cases, the numbers of layers of palisade cells have been counted and this figure used as a diagnostic character. Because in some plants the leaves growing in bright light may be thicker and have more layers of palisade cells than those leaves that have developed in the shade, this is not a sound diagnostic character and is clearly an effect of the environment.
Pharmacognosists (who, among other things, study plants and animals for natural products that might be applied in medicine) use a measurement called the ‘palisade ratio’. This is particularly useful in defining small leaf fragments in powdered leaf products. This measure indicates the number of palisade cells that can be seen beneath an epidermal cell in surface view. An average figure is produced after many cells are counted. A statistically sound count will produce a fairly reliable typification and hence identifica-tion of the material.
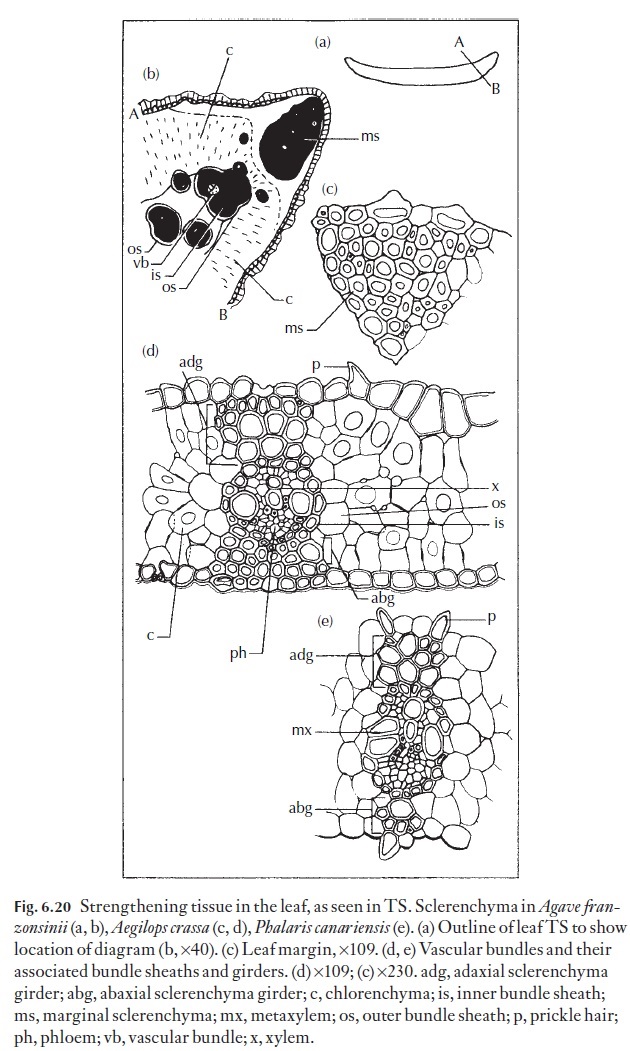
The arrangement of mesophyll cells may indicate whether a plant has the normal, C3 photosynthetic pathway (Fig. 6.20) or a C4 (Fig. 6.29a) photo-synthetic pathway. In Kranz, or C4 plants, the mesophyll consists of radiat-ing, elongated mesophyll cells surrounding a (usually) parenchymatous but often lignified bundle sheath, which, in turn, surrounds the vascular bun-dles. The radiating mesophyll is chloroplast-rich, and it is here that CO2 is incorporated into malate or aspartate as the first step in the C4 photosyn-thetic process. The parenchymatous bundle sheath cells on the other hand usually contain large, prominent, generally agranal chloroplasts. In C4 plants, malate or aspartate produced in the mesophyll cells is thought to be transported via the numerous plasmodesmata which occur at the interface between the mesophyll and bundle sheath cells, where CO2 is liberated and immediately fixed via the C3 photosynthetic cycle, becoming incorporated into sugars, other carbohydrates and amino acids essential to sustain the rapid growth common to C4 plants.
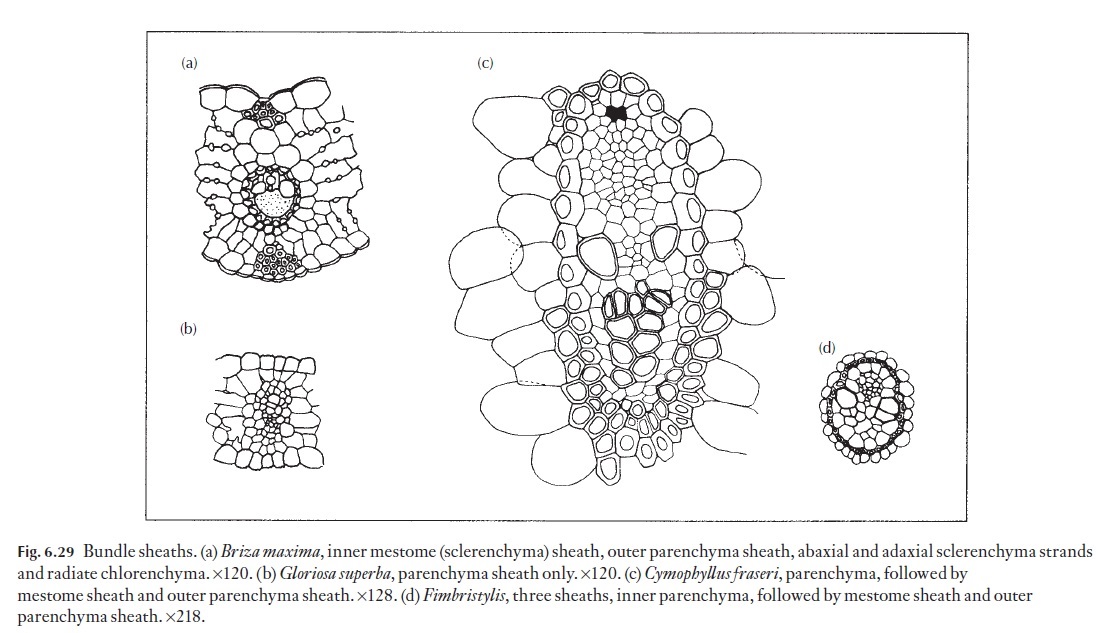
Among the Poaceae, there are a fairly large group of plants that are nei-ther C3, nor C4, but display intermediate leaf anatomy to that of the ‘typical’ C3 and C4 species. Here, it is the arrangement, structure and position of the mesophyll and bundle sheath chloroplasts that yields clues as to the degree of ‘intermediacy’. Whilst these C3–C4 intermediates are biochemically neither C3, nor C4, they seem to be able to follow a pathway that is depen-dent on several factors, including light intensity, air temperature, relative humidity, soil water availability and the nutritional status of the soil for example.
Some dicotyledonous foliage leaves contain a specialized, longitudinally orientated mesophyll, called the paraveinal mesophyll, which separates the upper palisade from the lower spongy mesophyll. As noted above, in many monocotyledonous plants, the mesophyll is not differentiated into spongy and palisade layers. The vascular bundles are surrounded by an initially parenchymatous bundle sheath, which may undergo lignification as the cells mature. There may be a specialized, concentric arrangement of the photosynthetic mesophyll surrounding the bundle sheath cells as in C4 plants.
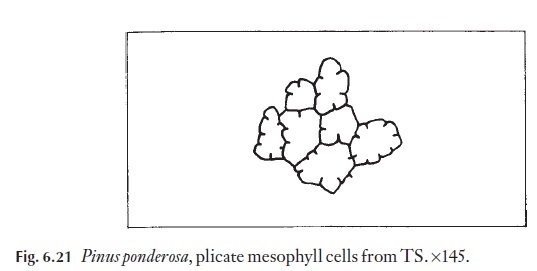
In many gymnosperms and some angiosperms the mesophyll cells are plicate, with inwardly directed wall foldings (Fig. 6.21). The infoldings increase cell wall surface area and probably therefore make up, to some extent, for the smaller number of chlorenchyma cells that are often found in such leaves.
Sclereids
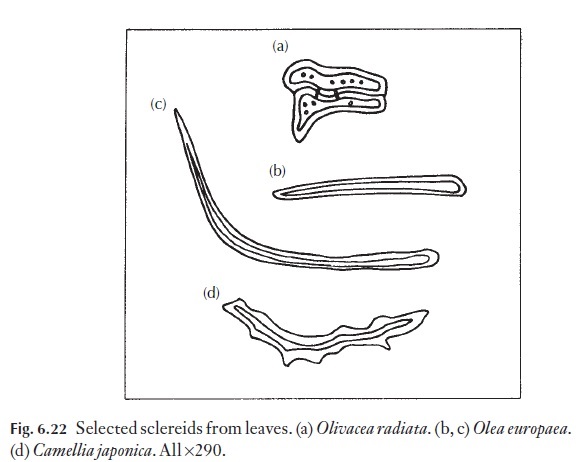
Sclereids can occur as isolated cells in the mesophyll, or in well-defined positions relative to other tissues such as within vascular bundles. Sclereids perform a mechanical supportive role, more especially in leaves, which lack well-developed girders or strands. They range in size and form, as described in the Glossary. Some of the types found, and the plants in which they occur, are shown in Fig. 6.22.
Air spaces
These may be present in the mesophyll, between veins. These are much larger and usually more formal than the air cavities between cells of the spongy mesophyll, and often form by the lysigenous (dissolving) or schizogenous (splitting) breakdown of thin-walled parenchymatous cells between veins. In monocotyledons, especially the grasses, the inter-cellular spaces are greatly reduced, particularly in more xerophytic species. Reduction of intercellular airspace volume is greatest in C4 xerophytic grasses.
Water storage cells
Water storage cells are large, colourless and thin-walled, and usually lack-ing in conspicuous cell contents. Sometimes areas of the wall may be thick-ened in such cells. Water storage cells occur in many families, notably those which have representatives growing in arid conditions.
Ergastic substances
Specialized cells in the mesophyll may be used in making identifications. Firstly, there are those cells containing ‘ergastic’ substances. These are products related to the physiological activity of the plant and may consti-tute stored food materials, such as starch, oil, protein and fat. They also include substances that cannot be related yet to a particular function. If the
This is a rather lazy way out of the problem, particularly since many of these substances are currently being identified as physiologically active by chemists. Chemists often do not know which cells of the plant contain them and it could be that some of the so-called ‘waste products’ are really important to the plant. Clearly, there is an obvious need for closer co-operation between morphologists and those extracting these potentially important and interesting plant products.
Crystals
Probably the best known of the ergastic substances, crystals, are very com-monly thought of as waste products, again without sound evidence. Crys-tals are usually composed of calcium oxalate and more rarely of calcium carbonate. Because they are of widespread occurrence, they are of limited value to the applied anatomist. However, some families have never been re-corded as having crystals, for example Juncaceae, the rush family. Others very frequently have a particular type, for example families within the As-paragales frequently have styloids (Fig. 6.23). Using this information, it should be possible to separate leaf fragments from families such as the Con-
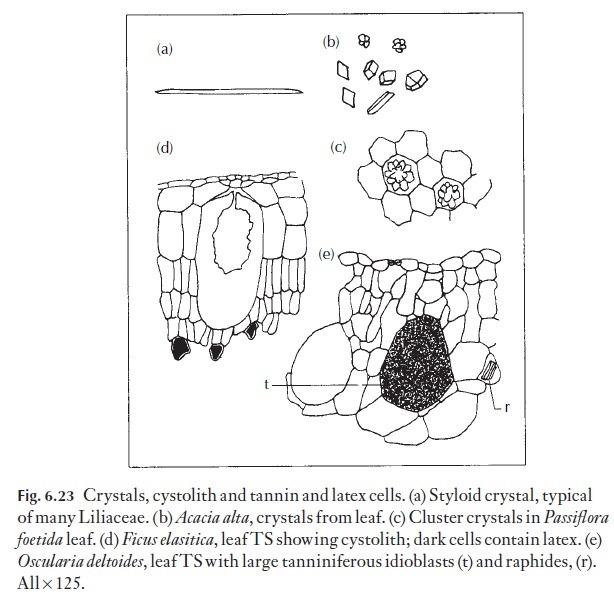
vallariaceae and Juncaceae. However, other monocotyledonous families, such as the Iridaceae, have crystals similar to those of the families in the As-paragales and such diagnostic characters must be used carefully and always in conjunction with others!
In the dicotyledons a particular ‘saddle-shaped’ or twin crystal is com-mon in Leguminosae (Fig. 6.23b) and its presence along with other features can help in distinguishing members of that family from others. Various prismatic and cluster crystals also shown in Fig. 6.25 have a very wide and scattered distribution through many families. Their presence in a fragment to be identified is only of real diagnostic value if they match exactly the type found in properly named reference material that has been narrowed down, using other characters, as probably being the species concerned.
Crystals can be associated with particular tissues, for example in the par-enchymatous bundle sheath surrounding the veins, or they may occur in
Sometimes there are no large crys-tals, but merely fine ‘crystal sand’ in the lumen of certain cells. Cystoliths are a special example of idioblasts; they occur in relatively few plants, for example Ficus elastica, and are illustrated in Fig. 6.23.
![]()
![]()
Silica bodies
These are similar in appearance to crystals. They normally occur in special cells (stegmata) next to fibres or other lignified tissues, or in the epidermal cells, particularly those near to fibrous cells associated with bundle sheaths. However, since silica bodies are amorphous and not crystalline in struc-ture, they can be distinguished from crystals by simple tests. In reality they are small opals! Silica bodies do not show birefringence (i.e. they do not shine brightly, as crystals do) when viewed between crossed polars in the polarizing microscope. In addition, they often turn pink when treated with a saturated solution of carbolic acid, and we know of no crystals that do that. If you should use this histochemical test, be careful to keep the carbolic acid off your skin and wear protective glasses!
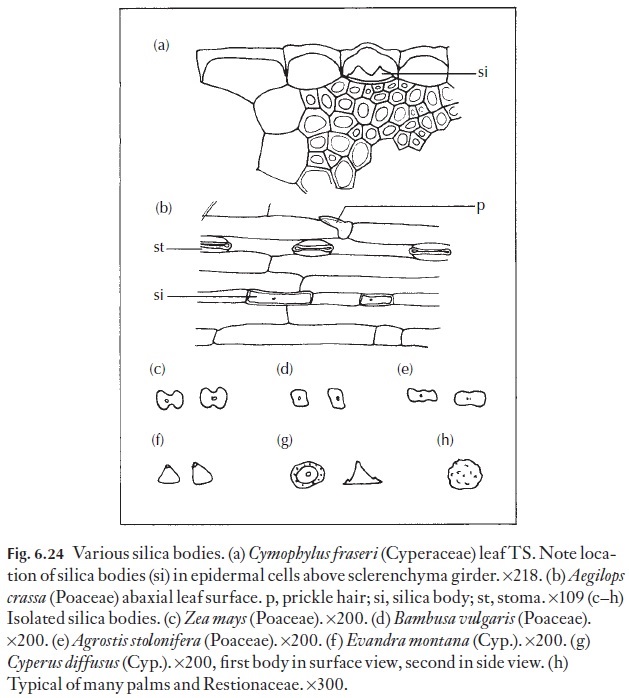
Silica bodies often occur in epidermal cells, usually one, but occasionally more to a cell, in a limited range of families. Because they are easy to see – it is worth examining a simple epidermal strip or scrape from one of the grass-es, the Cyperaceae, particularly Carex species or a palm leaf surface, for example in Borassus species. In the bamboos, as in Bambusa vulgaris, they are almost cuboid, as shown in Fig. 6.24. Those of Zea and Agrostis (dumb-bell-shaped to oblong) are also illustrated, together with some others from grasses or sedges that may be easily available to you.
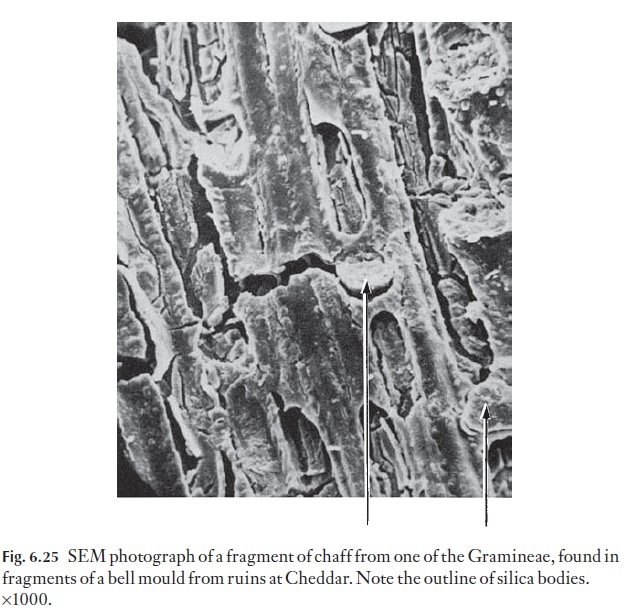
There are silica bodies of many shapes and sizes in the grasses and palms and extensive taxonomic use is made of them. Their form can be of help in the identification of fragments of cereal or grass that may have constituted part of the diet of an animal whose feeding habits are under investigation. They survive digestion and can be found in quite remarkable situations. For example, it was recent practice to use horse dung in the clay when bell founding and it was thought that medieval bell founders also used dung to reinforce the clay of their bell moulds. Fragments of bell moulds from ruins of a thirteenth-century chapel at Cheddar were examined for such evidence and there were leaf or chaff surface fragments together with silica bodies, probably of oats, (Fig. 6.25), which had survived being eaten, fired in the clay by the molten bell metal and then several hundred years of burial!
The silica bodies of sedges are cone-shaped or conical, with flat bases. They often have small satellite cones around them as shown in Fig. 6.24. No grasses have this type of silica body.
Closely related families can sometimes be distinguished through the presence or absence of silica bodies. For example, among the Juncales, the rush family, Juncaceae and the Centrolepidaceae, which is a very small fam-ily of semi-aquatic plants from the southern hemisphere, lack silica bodies. On the other hand Restionaceae, which are rush-like plants mainly from Australia and South Africa, typically have silica bodies shaped like small, spiky balls. In the Restionaceae, the silica bodies rarely occur in epidermal cells, but more frequently in stegmata, specialized cells with thickened inner and anticlinal walls and thin outer walls. The thickening is often lig-nified and sometimes also suberized. However, most species of Restionace-ae lack leaves, and as the silica bodies occur in cells in the stem this is probably not the place to be discussing them. However, the stem contains chlorenchyma and carries out many of the physiological functions of leaves in that family.
The function of silica bodies is not understood. It is thought that plants cannot prevent the uptake of silicon with other elements, and that silicon in excess is deposited in an inert form; hence the proximity of silica bodies to veins. However, this does not explain why many plants that must surely also take up silicon in excess do not form silica bodies. They do cause wear in teeth of grazing animals. However, in the process of co-evolution, such
animals have developed teeth that continue to grow during their lifetime, thus counteracting the deterrent.
![]()
![]() Ecologists have used silica bodies persisting in peat layers to determine the nature and species composition of earlier vegetation at a range of sites.
Ecologists have used silica bodies persisting in peat layers to determine the nature and species composition of earlier vegetation at a range of sites.
Tannins
Tannins generally have a scattered distribution through various plant fam-ilies. Polyphenolic substances are usually characterized by their reaction with ferric chloride solution, when they turn blue-black. Their chemical diversity is a phytochemical problem.
The presence of tannins in special cells or cell layers can, nevertheless, be used as a diagnostic character even if their chemical identity is not known. However, a word of caution is necessary. Tannin may appear at certain sea-sons in some plants, such as the Poaceae, so lack of tannins at a particular time of year is not a reliable feature, and the plants cannot be assumed to lack them totally. Some tanniferous idioblasts are illustrated in Fig. 6.23. Some Lithops species owe their mottled brown appearance to tannin cells. One very familiar family rich in tannin is, of course, the Theaceae to which the tea plant belongs.
The function of tannins is also little understood. They may act as an ultraviolet light shield, perhaps like the xanthophyll components in many other plants. Normally tannins occur in epidermal cells. High-intensity sunlight can damage chloroplasts, so such a ‘screen’ may impart physiologi-cal and ecological advantages. Additionally, the astringent taste (a warning of the harm they do in binding with the stomach wall?) may protect leaves from being eaten.
Aleurone grains
Aleurone grains may be present as may starch grains.
Related Topics