Chapter: Plant Anatomy:An Applied Approach: The leaf
Loading phloem in leaf
Loading phloem
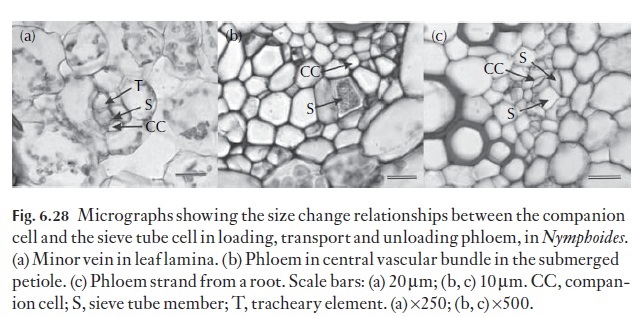
Within the leaf, the phloem is responsible for two distinct physiological activities. Firstly, the actual process whereby the sieve tubes are loaded (phloem loading), and secondly, they are involved with the transport of the loaded assimilates from the source to sinks in other parts of the plant (phlo-em transport). Clearly, the physiological activities of the sieve element– companion cell, the sieve element–transfer cell, or the relationship of the sieve elements to intermediary cells, in angiosperms, or the sieve cells to albuminous and other contiguous parenchyma cells, may influence the structure of these important cells within the leaf profoundly. Cross-sections of leaves, particularly of the minor veins in dicotyledonous, and of many monocotyledonous foliage leaves, reveals that the sieve element– companion cell complex (where the companion cell is clearly recognizable) consists of larger diameter companion cells and relatively narrow diameter sieve elements. Figure 6.28 illustrates this point for Nymphoides, where the sieve tube diameter in the smallest minor veins is approximately 6 µm and the companion cell is much larger, at approximately 30–35 µm in width. The companions cells and, for that matter, intermediary cells, (in the phlo-em of leaves of Coleus species for example) usually have a dense cytoplasmic matrix, and contain large populations of mitochondria, and as illustrated in Fig. 6.28, are much larger than their associated sieve tube members, reflecting their role in the phloem loading process.
Plasmodesmatal frequencies vary between the cells along the entire phloem loading pathway, i.e. from the mesophyll cells to the functional vas-cular parenchyma including companion cells, intermediary cells and albu-minous cells. In particular, plasmodesmatal frequencies (assuming that all plasmodesmata are functional) may strongly influence both the mode and the rate at which phloem loading occurs. The presence of plasmodesmata between cells is an indicator of potential symplastic loading, whilst the absence of plasmodesmata is an indication of a potential apoplastic phloem-loading pathway.
Sieve element loading may therefore be either symplastic or apoplastic. Whether loading is symplastic or apoplastic, there is ultrastructurally a great difference between the companion cell–sieve tube complex in the leaf, the stem and the root (Fig. 6.28). Oparka and Turgeon (1999) have suggested that the cell interactions between the sieve element and its companion cell must rank among the most complex and mysterious – they suggest that the companion cell functions as a ‘traffic control centre’ facilitating many and varied transport processes across the companion cell–sieve tube interface.
Structural and functional specialization of companion cells
The term ‘companion cell’ is used to describe that cell, or group of cells, which is derived from the same mother (procambial) cell as the sieve tube member. However, the identification of the ‘companion cell’ may be prob-lematical in some species, more especially so in monocotyledons. By con-trast, transfer cells are relatively easy to identify, as they always have wall ingrowths that enhance uptake from the apoplast due to the vastly increased cell wall and plasmalemma surface area. Intermediary cells occur in several families, and function in the phloem-loading pathway by conversion of the sugars into larger molecules, through a ‘polymer trap’ mechanism which effectively concentrates larger polymers in the intermediary cells, increasing their concentration, and facilitating phloem loading via the intermediary cells. Photoassimilates and other substances usually accumu-late against concentration gradients in the phloem, a process known as loading. In mature leaves, the sieve element–companion cell complexes (SE–CCs) of minor veins, where loading occurs, are connected to sur-rounding cells by plasmodesmata. These pores appear to participate in loading in plants that translocate raffinose and stachyose, but in sucrose-and polyol-translocating species their function is less certain (Turgeon 2000). There is argument that large numbers of plasmodesmata between the SE–CCs and surrounding cells should cause dissolution of the concen-tration gradient unless the size exclusion limit of the pores is small enough to retain accumulated solute species. In leaves of willow, Salix babylonica L., a sucrose-translocating plant with a high degree of symplastic connectivity into the minor vein phloem, the sucrose concentration gradient is absent between mesophyll and phloem, leading to the conclusion that phloem loading does not occur. Once inside the SE–CCs, solute may be able to pass freely between sieve elements and companion cells, because they are also symplastically connected. However, Turgeon (2000) postulates that due to net flux into the sieve tubes in source leaves, a continual drain of metabolic intermediates out of companion cells should occur, and this transport step could be regulated in minor veins, to prevent continual loss of needed solute molecules to the translocation stream.
![]()
![]() In a recent study, Hoffmann-Thoma et al. (2001) studied the minor-vein ultrastructure and sugar export in mature summer and winter leaves of the three broadleaf-evergreen species Ajuga reptans varartropurpurescens L., Au-cuba japonica Thunb. and Hedera helix L. to assess temperature effects onphloem loading. Leaves of the perennial herb Ajuga exported substantial amounts of assimilate in form of raffinose-family oligosaccharides (RFOs). Its minor-vein companion cells represent typical intermediary cells, with numerous small vacuoles and abundant plasmodesmal connectivity to the bundle sheath. By contrast, the woody plants Hedera and Aucuba translocated sucrose as the dominant sugar, and only traces of RFOs were reported. Their minor-vein phloem possessed a layer of highly vacuolated cells within the vascular bundles, intervening between mesophyll and sieve elements, which were classified either as companion or parenchyma cells, depending on the location and ontogeny of these vacuolate cells. Both cell types showed symplasmic continuity to the adjacent mesophyll tissue although at a lower plasmodesmal frequency compared to the Ajuga intermediary cells. p-Chloromercuribenzenesulfonic acid did not reduce leaf sugar export in any of the plants, indicating a symplasmic mode of phloem loading.
In a recent study, Hoffmann-Thoma et al. (2001) studied the minor-vein ultrastructure and sugar export in mature summer and winter leaves of the three broadleaf-evergreen species Ajuga reptans varartropurpurescens L., Au-cuba japonica Thunb. and Hedera helix L. to assess temperature effects onphloem loading. Leaves of the perennial herb Ajuga exported substantial amounts of assimilate in form of raffinose-family oligosaccharides (RFOs). Its minor-vein companion cells represent typical intermediary cells, with numerous small vacuoles and abundant plasmodesmal connectivity to the bundle sheath. By contrast, the woody plants Hedera and Aucuba translocated sucrose as the dominant sugar, and only traces of RFOs were reported. Their minor-vein phloem possessed a layer of highly vacuolated cells within the vascular bundles, intervening between mesophyll and sieve elements, which were classified either as companion or parenchyma cells, depending on the location and ontogeny of these vacuolate cells. Both cell types showed symplasmic continuity to the adjacent mesophyll tissue although at a lower plasmodesmal frequency compared to the Ajuga intermediary cells. p-Chloromercuribenzenesulfonic acid did not reduce leaf sugar export in any of the plants, indicating a symplasmic mode of phloem loading.
The companion cell, or specialized intermediary cell, are thus physio-logically significant in facilitating and regulating phloem loading, which appears in all species to be regulated in the minor (or small) veins of leaves. In the Magnolid Liriodendron tulipifera, plasmodesmatal frequencies lead-ing into minor vein companion cells are higher than in species known to ![]()
![]() load via the apoplast. According to Goggin et al. (2001), the companion cells are not specialized as ‘intermediary cells’ as they are in species in which the best evidence for symplastic phloem loading has been documented. Furthermore, application of the inhibitor, chloromercuribenzenesulfonic acid was demonstrated to largely, but not entirely, inhibit exudation of radiolabelled photoassimilate. The findings of Goggin et al (2001) are therefore most consistent with the presence of an apoplastic component to phloem loading in this species, contrary to speculation that the more basal members of the angiosperms load by an entirely symplastic phloem loading mechanism. Sucrose transporters are postulated to load photosynthetically produced sucrose apoplasmically into the sieve elements. For example, su-crose transporters like SUC2 have been localized in the companion cells (see Tazz and Zeiger, 2002). Mutant Arabidopsis plants containing DNA in-sertions in the gene encoding SUC2 have been recently identified which in the homozygous state, result in stunted growth, retarded development and sterility (Gottwald et al. 2000). Source leaves of mutant plants accumulate starch, and radiolabelled sugar was not efficiently transported to sinks such as roots and inflorescences.
load via the apoplast. According to Goggin et al. (2001), the companion cells are not specialized as ‘intermediary cells’ as they are in species in which the best evidence for symplastic phloem loading has been documented. Furthermore, application of the inhibitor, chloromercuribenzenesulfonic acid was demonstrated to largely, but not entirely, inhibit exudation of radiolabelled photoassimilate. The findings of Goggin et al (2001) are therefore most consistent with the presence of an apoplastic component to phloem loading in this species, contrary to speculation that the more basal members of the angiosperms load by an entirely symplastic phloem loading mechanism. Sucrose transporters are postulated to load photosynthetically produced sucrose apoplasmically into the sieve elements. For example, su-crose transporters like SUC2 have been localized in the companion cells (see Tazz and Zeiger, 2002). Mutant Arabidopsis plants containing DNA in-sertions in the gene encoding SUC2 have been recently identified which in the homozygous state, result in stunted growth, retarded development and sterility (Gottwald et al. 2000). Source leaves of mutant plants accumulate starch, and radiolabelled sugar was not efficiently transported to sinks such as roots and inflorescences.
Related Topics