Chapter: Modern Medical Toxicology: Chemical Poisons: Heavy Metals
Mercury - Chemical Poisons
Mercury
Synonyms
Quicksilver;
Liquid silver.
Physical Appearance
· Metallic or elemental mercury (Hg°) is a heavy, silvery liquid (Fig 9.13) which is per se non-toxic, but vapourises at room temperature to give off a toxic vapour: mercuric mercury. Volatilisation is greatly enhanced by heating. In its solid state, mercury is a tin-white, ductile metal that is malleable enough to be cut with a knife.

·
Salts or compounds of mercury may be
inorganic or organic in nature. Inorganic salts are of two types—mercuric
(bivalent, i.e. Hg++ ) and mercurous (monovalent, i.e.
Hg+) . Common mercuric salts include mercuric chloride (corrosive sublimate) which is a white
crystalline corrosive powder, mercuric oxide which is a red crystalline powder
that turns yellow when treated with caustic soda or potash, and mercuric
sulfide (vermilion or sindoor) which is a red crystalline
powder. The most importantmercurous salt is mercurous chloride (calomel) which is fibrous and heavy.
·
Organic mercurials are generally
more toxic and comprise mainly compounds such as phenyl and methoxymethyl mercury,
and alkyl compounds such as ethyl and methyl mercury. Mercurochrome is also an
organic mercurial and exists as iridescent green scales readily soluble in
water. The most toxic compound of mercury is methyl mercury.
Uses
·
Listed in Table 9.6.

Uses/Sources
·
Breaking of mercury fluorescent
light bulbs, heating of
Uses/Sources mercury-gold amalgams in order to extract gold, and the use
of mercury-containing latex paint or building materials.Ingestion or handling
of liquid mercury following breakage of thermometers or other
mercury-containing devices.
·
Insertion or removal of dental
amalgam restorations can generate mercury vapour or respirable particulates.
Bruxism, chewing, and tooth brushing may increase amalgam release of mercury
vapour.
·
Occupations which have the greatest
exposure to mercury vapours include mining and processing of cinnabar ore, the
chloralkali industry, and occupations in which mercury- containing instruments
or materials are manufactured or handled.
· Dietary exposure to mercury (in the form of methyl mercury) from consumption of fish, shellfish and marine mammals.
Usual Fatal Dose
■■ The
amount of ingested mercury that would be fatal to a man is estimated at 100
grams.
■■ Mercuric
chloride: 0.5 to 1 gm/70 kg
■■ Mercurous
chloride : 1.5 to 2 gm/70 kg
Toxicokinetics
After inhalation, elemental mercury
is readily absorbed through the alveolar membrane and enters the blood stream.
Ingestion of mercury salts is asociated with slower rate of absorption. Mercury
is rapidly converted to mercuric ions (Hg++) in the blood which can lead to
renal tubular damage during excre-tion. In the central nervous system, mercury
acts mainly upon cerebellum, temporal lobe, basal ganglia, and corpus callosum.
Both
organic and inorganic mercurials can be absorbed through intact skin.
Clinical Features
Poisoning
with elemental mercury and inorganic salts:
Acute poisoning—
––
Inhalation:
--
This usually occurs while heating metal in a closed room, or following gold
refining in an enclosed area.
--
Symptoms (which may be delayed upto 4 hrs) include dyspnoea, cough, fever,
headache, chills, GI disturbances, metallic taste, and blur-ring of vision.
Stomatitis, swelling of the sali-vary glands and gingivitis may develop within
a few days of acute exposure to mercury. Teeth may become loose due to gum
inflammation.
-- In severe cases there may be non-cardiogenic
pulmonary oedema, dyspnoea, convulsions, etc.
-- Sometimes manifestations similar to Kawasakidisease (mucocutaneous lymph node syndrome)are seen especially in children, which may be mistaken for scarlet fever: conjunctival congestion, fever, reddened palms and soles.
––
Ingestion:
-- Small quantities of elemental mercury
usually cause no harm on ingestion. Sometimes even a relatively large amount
may pass out of the body uneventfully (egged on by a mild laxative). It is
however advisable to take x-rays to monitor the progress of the elemental
mercury through the GI tract.
-- Ingestion of mercuric salts produces
corrosion leading to abdominal pain, vomiting, diarrhoea, and shock. The mucosa
of the GI tract usually appears greyish. There may be haematemesis. In severe
cases there is onset of renal failure, pulmonary oedema, and coma. Urine may
appear pinkish.
-- Ingestion
of button (or disc) batteries (Fig 9.16)
poses special problems since they contain a variety of caustic substances apart
from mercury. These batteries are used in hearing aids, watches, calculators,
and some hand- held computer games. They range in size from 7 to 25 mm and can
easily be swallowed by children. Each such battery contains a heavy metal
(usually mercury) along with a variety of caustic alkalies, espe-cially sodium
or potassium hydroxide.

»» In the majority of cases of button
battery ingestion there are no serious consequences, and the object usually
passes out in the stools quite uneventfully over a period of 1 to 4 days,
However if the contents of the battery leak out during transit, there can be
production of burns by the caustic alkalies, and a theoretical risk of mercury
poisoning (so far unreported).
»» Management:
⌂⌂ Airway assessment and
stabilisation. ⌂⌂ Antero-posterior and lateral chest radio-graphs that
visualise the neck, chest, and abdomen.
⌂⌂ Patients with batteries in the airway or lower respiratory tract require emer-gency removal via bronchoscopy.
⌂⌂ If the battey is visualised in
the oesoph-agus, it requires immediate endoscopic removal by forceps or magnet.![]()
⌂⌂ Intact batteries located past the
stomach in asymptomatic patients can be followed up by serial stool
examina-tions, checking for battery passage.
⌂⌂ If the battery fails to pass the
pyloric region within 48 hours, endoscopic retrieval may be necessary.
⌂⌂ The use of polyethylene glycol
solution in whole bowel irrigation can be consid-ered in patients with poor
mobility of batteries in the GI tract.
––
Injection:
·
Subcutaneous or intramuscular injections of elemental
mercury may cause abscess forma-tion wih ulceration, extruding tiny droplets of
mercury.
·
Intravenous injection can result in mercu-rialism characterised by thrombophlebitis,granuloma
formation, and pulmonary embo-lism. Repeated haemoptysis is a characteristic
feature.
·
Intra-arterial injection can result inadvertently from
arterial blood gas sampling with syringes which contain liquid mercury as an
anaerobic seal, or from arterial pressure monitors which employ a liquid
mercury manometer connected directly to the intra-arteial needle. Leakage of
mecury into arterial blood results in peripheral embolisation with ischaemia,
and sometimes frank gangrene. There may also be abscess formation and
ulceration. X-ray usually reveals multiple, tiny spheres in the veins draining
the entry site. Mercury globules may also be seen in various organs.
Chronic
poisoning (Hydrargyrism)—
––
Inhalation:
--
Tremor: It is one of the classical and most consistent manifestations of
chronic mercury poisoning and is sometimes referred to as the Danbury tremor. It begins in the hands
and isof a coarse, intentional type, interspersed with jerky movements. Later
it progresses to the lips, tongue, arms, and legs. The advanced condition is
referred to as Hatter’s shakes,* when
the tremor becomes so severe that daily activities involving some delicacy of
movement become grossly impaired, e.g. shaving, writing, holding a tumbler or
spoon, etc. The most severe form of the condition is referred to as concussio mercu-rialis when literally no
activity is possible. Evenyears after exposure to mercury has ceased, tremor
may persist.
--
Ataxia, reeling gait. Fasciculations of the tongue and legs have been
reported in paediatricpatients after several months of exposure to elemental
mercury.
--
A Parkinsonian syndrome with resting and intention tremor, bradykinesia
and cogwheel rigidity has been reported after long-term exposure to elemental
mercury vapours. The syndrome improved after penicillamine chela-tion.
--
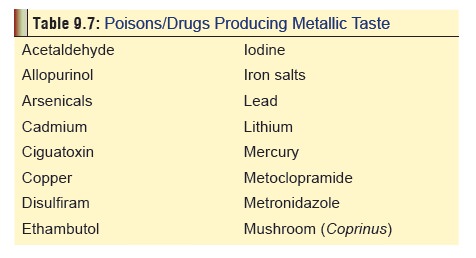
Metallic taste (Table 9.7),
anorexia, nausea, increased salivation.
--
Gingivitis, halitosis, blue line on gums (similar to the Burtonian line of plumbism).
--
Erythematous macular or papular rashes are common with mercury vapour
poisoning. The rash usually involves the hands and feet. The trunk, axillae,
popliteal and antecubital fossae may also be affected.
-- Erethism: Another classic manifestation, it refers to a cluster of psychiatric symptoms including abnormal shyness, loss of self confidence, depression, irritability, amnesia, excitability, progressing in later stages to delirium with hallucinations, or suicidal melancholia, or manic depressive psychosis (mad hatter).*
-- Mercuria lentis: Characterised by the
brown reflex of anterior lens capsule of the eye. There may be fine punctate
opacities. Visual blur- ring is often present, and sometimes there is
concentric constriction of visual fields (“tunnel vision”). Mercuria lentis
usually indicates chronic exposure to elemental mercury rather than toxicity.
Diagnosis is made by slit lamp examination.
--
Renal damage results in membranous glomerulonephritis with hyaline casts
and fatty casts in the urine. It is more common in chronic ingestion of mercury
salts.
––
Ingestion:
·
Colitis.
·
Melanosis coli.
·
Dementia.
·
Tremor.
·
Renal failure.
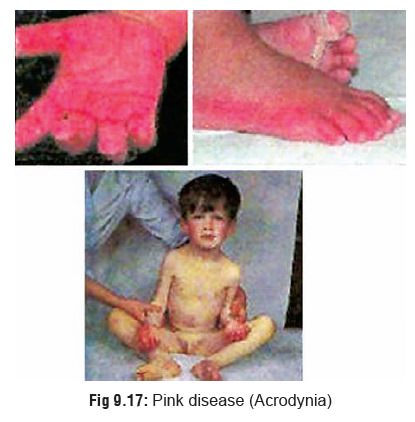
Acrodynia (Pink disease) (Fig 9.17): This is seen mainly in
children. Formerly most cases were related to chronic use of teething powders
containing mercurous chloride. But almost any form of chronic mercury exposure
can cause this condition including inhalation of elemental mercury vapour or
cutaneous application of ammoniated
mercury ointments. The onset is usually insidious with anorexia, insomnia,
profuse sweating, skin rash, and photophobia. The hands and feet become puffy, pinkish, painful, paraesthetic, perspiring and peeling (the child pees!).
Teeth may be shed, with ulcerationof gums. In older children and adults the
disease is milder, and is characterised by antisocial behaviour, insomnia,
aching extremities, and alopecia. Today the incidence of childhood acrodynia is
uncommon since the use of mercu-rial teething powders and diaper rinses has
been abandoned.
Exposure to mercury at an early age
is suggested as a possible factor in the aetiology of autism, as per recent
studies. According to these inves-tigators, the reported increased rates of
autism in some Western countries over the last few decades could be associated
with increased exposure to mercury through infant vaccines containing thimersol
(also spelt thimerosal or thiomersal), dental amalgams, or RhoD immu-noglobulin
injections that the mother received during pregnancy.
B. Poisoning with organic
mercurials:
·
Features are related mainly to the CNS and include
dysarthria, ataxia, paraesthesias, neuropathies, dimin-ished auditory and
visual acuity, mental deterioration, and chorea.
·
A special type of organic mercurial poisoning relates to
food poisoning through methyl mercury which has caused terrible tragedies in
the past, and may well cause more havoc in the years to come, unless measures
are devised to curb its far reaching effects. Between 1953 and 1970, on the
island of Kyushu around Minimata Bay in Japan, more than 2000 people were
diagnosed to be suffering from a curious cluster of neurological symp-toms
comprising paraesthesiae, narrowing of vision, dysarthria, diminution of
hearing, amnesia, ataxia, staggering gait, weakness, and emotional instability.
Some developed paralysis and became stuporous, and out of all the people
afflicted nearly a hundred died. The condition came to be known as the Minimata
disease (Fig 9.18), and
intensive investigations pointed to one inescapable conclusion: it was caused
by consump-tion of fish contaminated with methyl mercury, which originated from
a nearby vinyl chloride plant. The most severely affected victims were actually
infants who had been exposed in utero.

·
In 1964, a similar outbreak of poisoning was reported from
another part of Japan: Niigata along the Agano river. Forty three cases were
diagnosed as having the Minimata disease out of whom six died. Then came the
shocking tragedy in Iraq in 1971–72, when 500 people died out of a total of
6530 victims due to consumption of imported wheat and barley meant for sowing,
treated with methyl mercury. Nearly 95,000 tonnes of seed grain treated with
methyl mercury was baked into bread.
· Unlike inorganic mercury compounds, methyl mercury is a subtle, difficult to detect, long lasting poison. When large quantities of industrial waste and agricultural fungicides containing mercury are released into the ocean apart from volcanic discharges, methyla-tion of this relatively inoffensive metal results in the production of methyl mercury which then enters the algae-fish-human food chain.
This biological methyla-tion is accomplished
by a deep sea bacterium called methanobacterium
omelanskii . These bacteria areconsumed by plankton which in turn are eaten
by fish. When human beings partake of such contaminated fish, the scene is set
for a tragedy.
·
In the human body, methyl mercury is bound by haemoglobin
and circulates in this form in the blood-stream for several weeks or months.
Excretion is very slow and the estimated half life in man is 70 days, while in
fish it is 200 days. It passes easily into the CNS where it selectively and
irreversibly damages the cells of the granular layer of cerebellum and cerebral
cortex. Methyl mercury is especially injurious to the CNS of infants and
children.
Diagnosis
· X-ray.
· Blood mercury level: Flameless atomic absorption
spec-trometry is best for deducing this. Normal level is less than 3 mcg/100
ml. Symptoms of toxicity may occur at blood mercury concentrations of 5 mcg/100
ml or greater. Symptoms do not always correlate with blood mercury levels.
·
Urine mercury level: Urinary mercury is the best
biolog-ical marker for chronic elemental or inorganic mercury exposure. Urinary
mercury concentrations are also useful for assessing the response to chelation
therapy. Normal level is less than 10 to 15 mcg/100 ml. Signs and symp-toms of
toxicity may begin to occur at urinary mercury concentrations of 20 to 100
mcg/100 ml. Urinary mercury levels, however, often do not correlate with
clinical signs and symptoms of toxicity. There is a high degree ![]() of intraindividual variation in urine mercury levels. The
averaging of several urinary mercury determinations may be required. A 24-hour
urine collection is recommended.
of intraindividual variation in urine mercury levels. The
averaging of several urinary mercury determinations may be required. A 24-hour
urine collection is recommended.
· Hair analysis: Done by cold vapour atomic absorptionspectrometry.
External contamination can however vitiate the results.
Treatment
Acute
Poisoning:
Metallic mercury and inorganic
compounds—
––
Inhalation:
--
Supportive measures. -- Chelation
–– Ingestion:
--
In elemental mercury ingestion, take x-ray and repeat it to study the
progression. If mercury gets lodged in the appendix, perform appen-dectomy.
-- Administer laxatives.
--
Demulcents for corrosive compounds such as mercuric chloride.
--
Stomach wash: It may be advisable to add egg white or 5% albumin or just plain
milk to the lavage fluid to bind the mercury.
--
Chelation. –– Injection:
-- If there is abscess formation, perform repeated
incisions to remove the mercury. If the globules are very minute and widely
distributed in the intercellular spaces, excise the affected tissue.
--
Monitor the CNS and renal functions for evidence of toxicity.
--
Mercuric salts are relatively well adsorbed by activated charcoal.
-- Chelation.
Organic mercurials
–– Supportive measures.
–– Chelation is not very effective.
––
In severe manifestations with acute renal failure resulting from any type of
exposure, the following may be tried: haemodialysis, haemofiltration, or plasma
exchange. Haemoperfusion is said to be ineffective.
Chronic
Poisoning:
Chelation therapy—
––
BAL (British Anti Lewisite)
--
100 mg by deep IM, every 4 hours for 48 hours, followed by 100 mg every
8 hours for 8 to 10 days.
OR
––
DMPS ( 2,3 DiMercapto Propane-1-Sulfonate)
-- 5 mg/kg IV, or 6 infusions of 250
mg/day, followed by 100 mg orally twice a day for 24 days.
OR
–– DMSA (Meso 2,3 DiMercapto
Succinic Acid, or Succimer)
--30 mg/kg/day orally for 5 days,
followed by 20 mg/day for 14 days.
OR
––
D-Penicillamine
-- 250 mg qid, for adults, (20
mg/kg/day) for 5 to 10 days.
Supportive measures.
Autopsy Features
·
In death due to acute mercury
poisoning, the mucosa of the mouth, throat, oesophagus, and stomach appears
greyish in colour with softening and superficial corrosion. There may be
haemorrhagic points.
·
If there had been survival for a few
days, the large intestine may reveal ulceration.
·
Kidneys are often pale and swollen
due to oedema of renal cortex. Microscopy usually demonstrates necrotic changes
in the renal tubules.
Forensic Issues
·
Mercury and its compounds constitute
important industrial, agricultural, and occupational toxic hazards. In the past
when mercury was used in its various forms for the therapy of a wide variety of
ailments including syphilis, iatrogenic poisoning was common. Even today
calomel (mercurous chloride) is sometimes recommended as a laxative. Prolonged
use can cause colitis dementia, tremor, and renal failure. Acrodynia resulting
from teething powders containing calomel has fortunately become a rarity.
·
The tragic consequences of chronic
industrial exposure to mercury have been outlined in the foregoing sections.
Domestic exposure though not often reported is today a distinct threat. Such
exposure may result from inadvertent mercury spills (broken thermometers), or
from home gold- ore processing.
·
An important area that must be
considered in relation to chronic mercury exposure is dentistry. Dental
amalgams used for fillings often contain mercury. Dental surgeons may get
chronically exposed due to this reason. Many dentists still knead the amalgam
mass in the palms of their hands. In squeezing the mass to express excess
mercury, droplets of the metal sometimes fall to the floor where they are allowed
to remain and vapourise. Significant concentrations may be detectable even in
the reception room environ-ment. Recent studies of mercury levels in dental
surgeons suggest that they are at high-risk for developing mercury related
polyneuropathies, psychopathies, and visual distur- bances. However patients
wih mercury amalgam fillings are not at risk even though such fillings may
remain intact for years.
·
Food poisoning (especially involving
fish) through mercury compounds can give rise to manifestations akin to the
Minimata disease.
·
A relatively new hazard concerns dry
cell batteries used in cameras, hearing aids, torches, and watches.
Related Topics