Chapter: Modern Medical Toxicology: Chemical Poisons: Heavy Metals
Lead - Chemical Poisons

Lead
Lead
is the commonest metal involved in chronic poisoning. It was one of the first
metals known to man and has been widely used during the last two thousand years
for domestic, indus-trial, and therapeutic purposes. Lead is abundant in soil,
being distributed throughout the earth’s crust.
Physical Appearances and Uses
Elemental lead exists as a highly lustrous, heavy, silvery-grey metal (Fig 9.6) with a cubic crystal structure that assumes a bluish tint as it tarnishes in air.

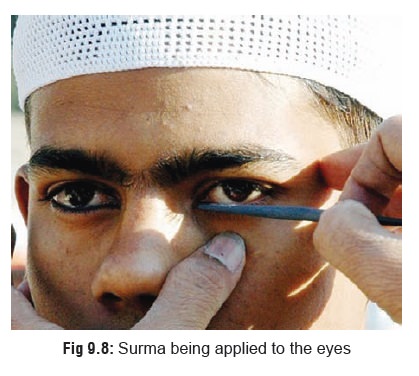
It is quite soft and malleable. Several of its salts occur as variously coloured powders or liquids and are used widely in industry and at home producing cumulative toxicity on chronic exposure. Lead acetate (sugarof lead) has been used in therapeutics,* lead carbonate (white lead) is still used in paints, lead oxide (litharge) is essential forglazing of pottery and enamel ware, and tetraethyl lead is mixed with petrol as an antiknock to prevent detonation in internal combustion engines. Among cosmetics, lead tetroxide is the most common compound in vermilion (“sindoor”) (Fig 9.7) applied by married Hindu women to the parting of their scalp hair, while lead sulfide is used as a collyrium (“surma”) (Fig9.8) for the eyes by Muslims.**

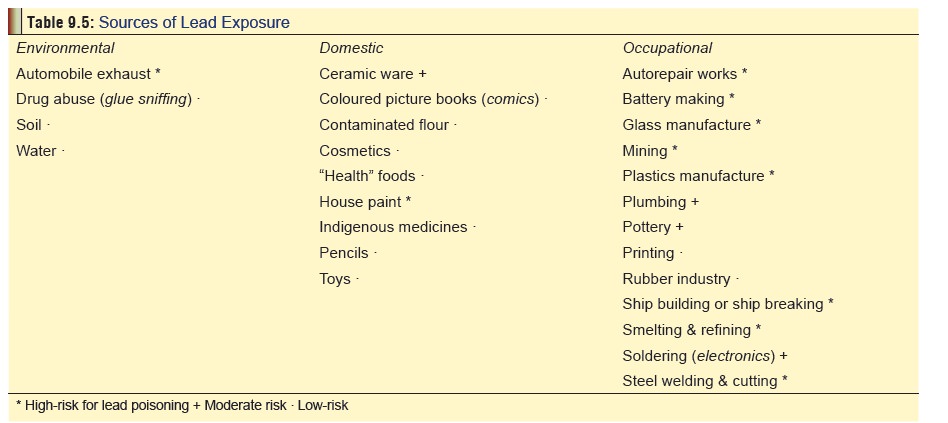
The
various situations in which lead salts are used resulting in chronic
occupational, environmental, or domesic exposure are listed in Table 9.5.
Summary
of some of the common non-occupational sources:
·
Candle with lead-containing wicks
·
Ayurvedic medicines
·
Paint
·
Retained bullets
·
Ink
·
Automobile storage battery casing;
battery repair shops
·
Ceramic glazes
·
Lead pipes
·
Silver jewellery workers
· Renovation/modernisation of old homes.
split
between ingestion and inhalation exposure routes. About 5–15% of ingested lead
is absorbed by adults with less than 5% retained. Children, however, absorb
approximately 50% of ingested lead and retain about 30%.
Usual Fatal Dose
This
is not really relevant to lead since acute poisoning is very rare. The average
lethal dose is said to be 10 gm/70 kg for most lead salts, while it is 100
mg/kg for tetraethyl lead.
Toxicokinetics
·
Lead is absorbed through all portals
of entry. Occupational exposure results mainly from inhalation, while in most
other situations the mode of intake is ingestion. Tetraethyl lead can be
absorbed rapidly through intact skin.
·
Following absorption, it is stored
in the bones as phosphate and carbonate. In children about 70% of total body
lead is skeletal, while in adults over 95% is in osseous tissues. Lead is drawn
to those areas of the skeleton which are growing most rapidly. These include
the radius, tibia, and femur, which are the most metabolically active. The
hyperminer- alisation is reflected in the form of densities which are the
classic “lead lines” observed on
x-ray (Fig 9.9).
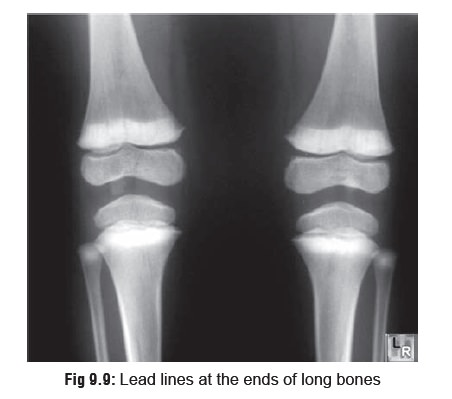
o The
width of the lead lines is related to the duration of exposure.
o Significant
amounts of skeletal lead are released from bone into the blood stream
periodically resulting in symptoms of toxicity. The conditions favouring this
include acidosis, fevers, alcoholic intake, and even exposure to sunlight.
·
Absorbed lead which is not retained
in the body is excreted primarily in the urine (about 65%) and bile (about
35%).
Mode of Action
· Lead combines with sulfhydryl enzymes leading to interference with their action.
·
It decreases haeme synthesis by
inactivating the enzymes involved such as aminolaevulinic
acid dehydrase,aminolaevulinic acid synthetase, coproporphyrinogen oxidase (or decarboxylase), and ferrochelatase. This resultsin anaemia.
·
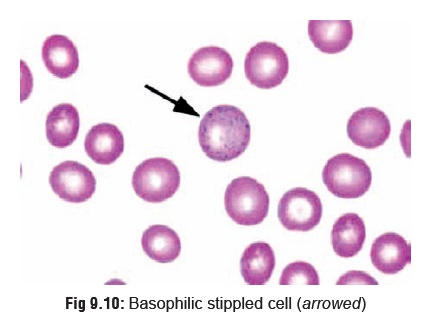
Lead increases haemolysis as a
result of which immature red cells are released into circulation such as
reticulocytes and basophilic stippled
cells (the result of aggregation of ribonucleic acid due to inhibition of
the enzyme pyrimidine-5-nucleotidase which normally eliminates degraded RNA) (Fig 9.10).
· In the CNS, lead causes oedema and
has a direct cytotoxic effect leading to decreased nerve conduction, increased
psychomotor activity, lower IQ, and behavioural/learning disorders. Children
are especially susceptible. The highest brain concentrations of lead are found
in hippocampus, cerebellum, cerebral cortex, and medulla.
· Lead also has deleterious effects on the CVS (hypertension and myocarditis), kidney (nephritis),* and reproductive organs (infertility). Lead nephropathy after chronic lead exposure has been well described. Interstitial nephritis, reduced glomerular filtration rate, and nonspecific proximal tubular dysfunction are typical. In addition, lead can decrease uric acid renal excretion, thereby raising blood urate levels and predisposing to gout (saturnine gout). Elevated urinary levels of N-acetyl-3-D-glucosaminidase and beta-2-microglobulin may serve as early markers of renal injury.
Permissible lead intake and blood
levels:
·
There is much controversy over this
at the present time. Lead appears as a trace metal in virtually all foods and
beverages, though fortunately absorption from such sources is relatively low.
·
Adults ingest 300 mcg and inhale 15
mcg of lead approximately each day, of which only 10% is absorbed, but children
may absorb upto 50%.
·
An important source of food based
lead poisoning is the use of lead-soldered canned food and drink. While in USA
measures have been taken to ban lead soldering of cans, the Indian canned food
industry may still be persisting with lead soldered seams, though there is no
clear information on this.
·
Lead in drinking water may be
absorbed to greater extent than that in food. The concentration of lead which
may not really be very high in ground or surface water may progressively rise
as it passes through the distribu-tion system because of contact with lead
connectors, lead service lines or pipes, lead soldered joints, lead containing
coolers, and lead impregnated fixtures such as brass taps.
·
Because of the reasons mentioned,
minute quanti-ties of lead are always present in the blood of even normal
individuals. Only when the concentration is high, do features of intoxication
begin to manifest. Today the accepted upper level for blood lead (BL) is fixed
as 35 mcg/100 ml. However there are reports that adverse effects especially on
the haematopoietic system can occur at levels as low as 10 mcg/100 ml.
Neurobehavioural disorders in children can occur at BL as low as 25 mcg/100 ml.
Hence, the current trend is to consider even levels as low as 10 mcg/100 ml as
unacceptable, especially in children.
Clinical Features
1.Acute poisoning—
·
This is rare. Many reported cases of
acute poisoning may actually be exacerbations of chronic lead poisoning when
significant quantities of lead are suddenly released into the bloodstream from
bone.
·
Symptoms include metallic taste,
abdominal pain, constipation or diarrhoea (stools may be blackish due to lead
sulfide), vomiting, hyperactivity or lethargy, ataxia, behavioural changes,
convulsions, and coma.
2. Chronic poisoning—
Mild Toxicity (BL 40 to 60 mcg/100 ml):
·
Myalgia
·
Paraesthesia
·
Fatigue
·
Irritability
·
Abdominal discomfort.
Moderate Toxicity (BL 60 to 100 mcg/100 ml) :
·
Arthralgia (especially nocturnal)
·
Muscular exhaustibility
·
Tremor
·
Headache
·
Diffuse abdominal pain
·
Anorexia, metallic taste, vomiting
·
Constipation
·
Weight loss
·
Hypertension.
Severe Toxicity (BL more than 100 mcg/100 ml) :
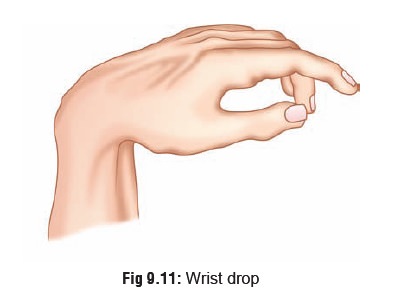
·
Lead
palsy: wrist drop (Fig 9.11)
or foot drop.
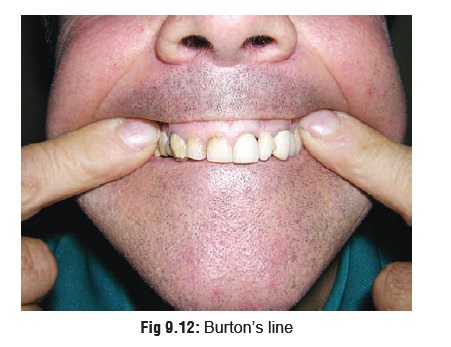
·
A bluish black lead line on gums (Burton’s
line)(Fig 9.12).*
·
Lead
colic: intermittent severe abdominal cramps. There may be
tenderness around the umbilicus.
· Lead encephalopathy: It is more common in children and is often associated with organic lead toxicity, especially tetraethyl lead or TEL which is lipid soluble and is distributed widely in lipophilic tissues such as the brain. TEL is metabolised to triethyl lead which is the major toxic compound. There is sudden onset of vomiting, irritability, headache, ataxia, vertigo, convulsions, psychotic manifestations, coma, and death. Mortality rate is around 25%. Even if recovery occurs, there is often permanent brain damage manifesting as mental retardation, cerebral palsy, optic neuropathy, hyper-kinesis, and periodic convulsions.
Note:
■■ Facial pallor,
especially circumoral is said to be a char-acteristic feature of chronic lead
poisoning and is due to vasospasm, though anaemia may contribute to a
significant extent.
■■ The anaemia that is
encountered in plumbism is similar to that due to iron deficiency, i.e. it is
hypochromic and microcytic in type; but anaemia due to the latter is neither
associated with reticulocytosis, nor are basophilic stippled cells seen.
Anaemia is not a prominent feature in organic lead poisoning. Basophilic
stippled cells are also not commonly encountered.
■■ Increasing blood
lead levels in children have been corre-lated with hearing impairment,
developmental delay, aggressive, hyperactive and antisocial behaviour, visual
problems, and growth retardation.
■■ Lead is transferred
across the placenta. It can affect reproduction in males and females, and
affects neurode-velopmental milestones in children with both prenatal and
postnatal exposure. Lead poisoning during pregnancy has been associated with
prematurity, low birth weight, and impaired foetal growth.
■■ Lead is increasingly being implicated as a carcinogen. Some
lead salts have produced tumours in experimental animal studies.
Related Topics