Chapter: Modern Medical Toxicology: Chemical Poisons: Heavy Metals
Iron - Chemical Poisons
Iron
Physical Appearance
·
Metallic iron is silvery white in
colour, occurring naturally as haematite, magnetite, etc. and usually causes no
problems. In fact it is an essential element and deficiency results in anaemia.
Even if there is more than the required intake daily, the excess is excreted.
But in some individuals with inborn errors, even normal dietary iron can cause
toxic effects due to accumulation, e.g. haemochromatosis (bronze diabetes).
·
Various iron salts are administered
therapeutically in indi-viduals with iron deficiency anaemia which can result
from a wide variety of causes. Iron poisoning is related in most instances to
overdose of such salts. One of the commonest is ferrous sulfate (green vitriol) which occurs as bluish
greenis ferrous sulfate (green vitriol)
which occurs as bluish green crystals (Fig
9.19). Iron (ferric) oxide, i.e. rust does not cause iron poisoning.

Uses/Sources
·
Dietary Sources:
·
The required daily amount of iron of
10–20 mg for adults is supplied through average diet. The required intake
increases to 25–30 mg in pregnancy. The average daily intake for adults is 15
mg. Environmental Sources:
· Iron is found in 5.1% of the earth’s crust. It is the second most abundant metal, and the fourth most abundant element. It is believed that the earth’s core consists mainly of iron.
Industrial
uses—
·
Iron is primarily used in powder
metallurgy and serves as a catalyst in chemical reactions.
·
Iron is a component of carbon
steels, cast iron, high-speed steels, high-strength low-alloy steels, manganese
alloy steels, and stainless steels.
·
Steel is the most important alloy of
iron. It contains 0.25–2% of carbon. Alloyed with carbon (C), manganese (Mn),
chromium (Cr), nickel (Ni) and other elements, iron is used to form steel.
·
Wrought iron is almost pure iron.
·
Iron uses include magnets, dyes,
pigments, and abrasives.
Biological uses
·
Iron is essential to life. It is a
constituent of biolog-ical pigments such as haemoglobin, cytochromes and
ferrichromes.
Usual Fatal Dose
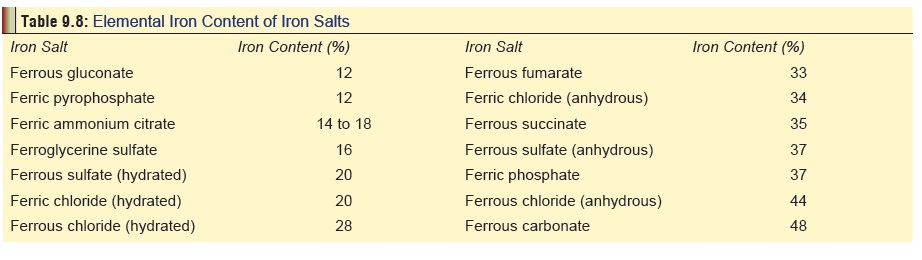
·
Commonly used iron salts in
therapeutics along with respective iron content are mentioned in Table 9.8.
·
The amount of iron in a particular
iron salt (e.g. sulfate, gluconate, fumarate, etc.) is not the same. Take the
total molecular weight of iron in the compound, and divide it by the molecular
weight of the compound and multiply by 100. Multiply the total number of
milligrams of the compound ingested by the percentage of iron in the compound.
Another fast method to remember the approximate amount of iron in a preparation
is FSG:359. This means the amount of ferrous Fumarate divided by 3; Sulfate
divided by 5 and Gluconate divided by 9 is the amount of elemental iron in the
prepara-tion.
·
The usual fatal dose corresponds to
about 200 to 250 mg of elemental iron per kg of body weight. This can be
calculated from the percentage of elemental iron in a particular prepara- tion,
e.g. a single 150 mg tablet of anhydrous ferrous sulfate which contains 37% of
elemental iron will contain a total of 55 mg of elemental iron. But such
calculations can be misleading since serious hepatotoxicity can result at much
lower concentra- tions of iron in the body which can lead to death. In
practice, this can be as low as 60 mg of elemental iron/kg. Hence just a
handful of these tablets (15 to 20 in number), can be lethal to a young child.
![]()
Toxicokinetics
Iron
poisoning occurs when serum iron level exceeds the total iron-binding capacity
(TIBC), resulting in free circulating iron in the bloodstream.
Mode of Action
·
Free iron causes:
·
Massive postarteriolar dilatation
which results in venous pooling.
·
Increased capillary permeability
resulting in decreased plasma volume.
·
Oxidation of ferrous to ferric iron
releasing hydrogen ions. Subsequent hydration of ferric iron results in
metabolic acidosis.
·
Inhibits mitochondrial function
leading to hepatic damage, hypoglycaemia, and hypoprothrombinaemia.
·
Inhibits thrombin-induced conversion
of fibrinogen into fibrin.
·
Has a direct corrosive action on the
GI mucosa.
Clinical Features
·
Most cases occur in children. There
are 5 stages:
·
Stage
I (0.5 to 2 hours) includes vomiting, haematemesis, abdominal
pain, diarrhoea, haematochezia, lethargy, shock, acidosis, and coagulopathy.
Necrosis to the GI tract occurs from the direct effect of iron on GI mucosa.
Severe gastro- intestinal haemorrhagic necrosis with large losses of fluid and
blood contribute to shock. Free iron and ferritin produce vasodilatation that
may also contribute to shock.
·
Stage
II (after Stage I)
includes apparent recovery and may contribute to a false sense of security.
Observe closely. Stage III (2 to 12
hours after Stage I) includes
profound shock, severe acidosis, cyanosis and fever. Increased total peripheral
resistance, decreased plasma volume, haemo- concentration, decrease in total
blood volume, hypotension,
·
CNS depression, and metabolic
acidosis have been reported.
·
Stage
IV (2 to 4 days) includes possible hepatotoxicity, convulsions,
and coma. Thought to be a direct action of iron on mitochondria. Monitor liver
function tests and bilirubin. Acute lung injury may also occur.
o The
primary site of hepatic injury is the periportal areas of the hepatic lobule
(the principal site for hepatic regeneration), which may explain the increase
in mortality and poorer prognosis. Iron induced hepato- toxicity is a presumed
result of free radical generation and lipid peroxidation. Iron catalyses
hydroxyl radical formation (the most potent-free radical), which initiates
lipid peroxidation. Based on limited data, antioxidants may have a
hepatoprotective role in iron poisoning.
·
Stage
V (days to weeks) includes GI scarring and strictures. GI
obstruction secondary to gastric or pyloric scarring may occur due to corrosive
effects of iron. Evaluate with barium contrast studies. Sustained-release
preparations have resulted in small intestinal necrosis with resultant scarring
and obstruction.
These stages of iron poisoning may not occur in all
patients.
After massive overdose, patients may present in shock. With
less serious overdoses, the initial gastrointestinal symptoms may be the only
findings to develop even without treatment.
Diagnosis
·
X-ray: Like all other heavy metals,
iron and its compounds are radiopaque. However, chewable iron tablets and
liquid iron formulations are usually not visualised on x-ray. Completely
dissolved iron tablets/capsules may also not be radiopaque.
·
Serum iron level: Poisoning is
indicated if this exceeds 150 mcg/100 ml, and serious toxicity is usually
associated with levels beyond 500 to 600 mcg/100 ml. Peak levels are seen
around 4 hours after ingestion. Measuring the total iron binding capacity and
relating it to the serum iron level is often misleading and unreliable.
·
Total leucocyte count (TLC),
electrolytes, glucose, blood gas, clotting studies, liver function and renal
function tests are useful estimates.
·
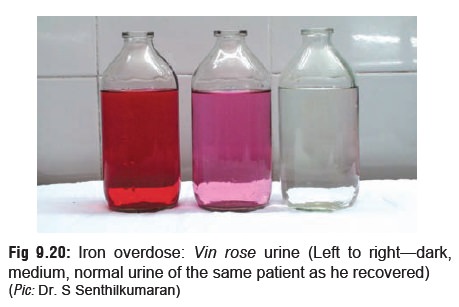
Chelation challenge test:
Desferrioxamine in a dose of 25 mg/kg (maximum 1 gm) is given IM. If the serum
iron has exceeded iron binding capacity, the excess iron is chelated to
desferrioxamine and the complex is excreted as a pinkish (vin rosé) colour in
the urine (Fig 9.20). But a negative result does not rule out iron poisoning.
·
Qualitative desferrioxamine colour
test (QDCT): 2 ml of gastric fluid and 2 drops of 30% hydrogen peroxide are
placed in 2 plastic tubes. 0.5 ml of solution of desferriox-amine (500 mg in 4
ml distilled water) is added into one tube and the resulting colour change is
compared with the other tube (control). If the test is positive, an orange to
red colour will develop in the tube in which desferrioxamine was added. The
test must be done within 2 hours of inges-tion of iron.
Treatment
·
Stomach wash with normal saline
performed gently may be of benefit in massive ingestions. Desferrioxamine must
not be used for lavage.
·
Activated charcoal is ineffective.
· Magnesium hydroxide solution (1%) administered orally may help reduce absorption of iron by precipitating the calcium carbonate containing antacids may safely be used in therapeutic doses to help reduce iron absorption.
·
Obtain serum iron levels,
creatinine, electrolytes, bloodhaemoglobin concentration, blood prothrombin
time, base line liver function tests, and arterial blood gases in seriously
poisoned patients.
·
Correction of hypovolaemia, and
metabolic acidosis.
Chelation therapy:
·
This is indicated in any of the
following situations:
o More
than one episode of vomiting or diarrhoea.
o Significant
abdominal pain, hypovolaemia, or acidosis.
o Multiple
radiopacities on abdominal radiograph.
o Serum
iron level greater than 350 mcg/100 ml.
·
Chelation can be done either with
desferrioxamine (parenteral) or
deferiprone (oral).
Dose (desferrioxamine):
·
Intravenous Dose: Administer by
continuous infusion at a rate of up to 15 mg/kg/hr. Faster rates or IV boluses
may cause hypotension in some individuals. Infusion rates up to 35 mg/ kg/hr have
been used in children with severe overdoses without adverse effects.
·
Intramuscular Dose: Administer 90
mg/kg, up to a maximum of 1 gm/dose, every 8 hours as are often experienced.
·
Total Daily Dose: The recommended
total intra- venous or intramuscular daily dose should not generally exceed 6
grams.
·
Duration of Infusion: Duration of
infusion is guided by the patient’s clinical condition. Patients with moderate
toxicity are generally treated for 8 to 12 hours, those with severe toxicity
may require desferrioxamine for 24 hours or longer. Patients should be
re-evaluated for evidence of recurrent toxicity (hypotension, metabolic
acidosis) several hours after the infusion is discontinued. Infusion duration
of greater than 24 hours has been
associated with the development of ARDS.
·
Therapy Endpoint/Colour Change:
Monitor urine for characteristic pink to orange-red colour (“vin rose”) indicating the excretion of
ferrioxamine (chelated iron) although frequently a urine colour change is not
seen. In patients who demonstrate a colour change, desferriox- amine therapy
may be discontinued when the urine loses the “vin rose” colour, indicating a decrease in concentration of
chelated complex, if the patient is generally asymptomatic.
Adverse Effects:
·
Sepsis: The use of desferrioxamine
in iron-over- dosed children has been associated with Yersinia enterocolitica septicaemia and mucormycosis. In such
circumstances desferrioxamine may have provided the iron siderophore complex
growth factor needed by the bacteria to induce overgrowth.![]()
·
Visual Toxicity: Continuous
intravenous administration of desferrioxamine, often in the presence of low
iron stores, has produced visual toxicity (decreased visual acuity, night
blindness, colour blindness, retinal pigmentary abnormalities). Visual toxicity
has also been associated in patients with rheumatoid arthritis and chronic
renal failure. The mechanism remains unclear.
·
Ototoxicity: In one study, some
patients receiving desferrioxamine had abnormal audio-grams, with a few
requiring hearing aids. Risk factors include desferrioxamine dose, duration of
therapy and the presence of a low serum ferritin.
·
Pulmonary Toxicity: A “pulmonary
syndrome” has been associated with high dose IV (10 to 25 mg/kg/hr)
desferrioxamine therapy for several days for acute and chronic iron overload
patients. Features include severe tachypnoea, hypoxaemia, fever, eosinophilia,
preceding urticaria, and pulmonary infiltrates.
·
Hypotension appears to be rate
related. One study has suggested that intravenous desferriox-amine be
administered at a dose NOT to exceed 15 mg/kg/hr. At present, a safe
administration rate has not been established and is based on empiric data.
·
Renal Toxicity: Elevated creatinine
levels and decreased creatinine clearances have been reported.
·
Continuous arteriovenous
haemofiltration (CAVH) may be helpful in severe poisoning.
·
Liver transplantation is the only
therapeutic avenue open in the presence of fulminant hepatic failure.
Autopsy Features
·
Haemorrhagic necrosis of gastric
mucosa. In ferrous sulfate poisoning, gastric contents may appear bluish green
in colour.
·
Hepatic and renal necrosis.
Forensic Issues
Acute iron poisoning has assumed
grave significance in recent years and cases of accidental poisoning are being
reported with alarming frequency in young children. Since most iron
preparations (syrups and tablets) are brightly coloured and pleasantly
flavoured, they constitute an irresistible, fatal attrac-tion for these
innocent victims. To compound the tragedy, in several instances the parents themselves
are ignorant about the toxicity of these preparations and tend to dismiss them
as “harmless vitamins”. It is imperative that public awareness be generated
about the treacherous lethality of iron preparations. Introduction of
childproof containers would be very effective in minimising inadvertent
ingestions by children as demonstrated by the Western experience.
Related Topics