Chapter: Introduction to Human Nutrition: Nutrition and Metabolism of Proteins and Amino Acids
Meeting protein and amino acid needs
Meeting protein and amino acid needs
Knowledge of the requirements for the specific indis-pensable amino acids and for total protein provides the basis for evaluating the relative capacity (or quality) of individual protein foods or mixtures of food protein sources to meet human amino acid requirements.
The two major determinants of the nutritional quality of food proteins are:
● the content of indispensable amino acids in the protein
● the extent to which the indispensable amino acids are available to the host metabolism.
Digestibility and intestinal amino acid metabolism
Traditionally, the assessment of the availability of dietary proteins and amino acids under practical con-ditions has been based on “apparent digestibility,” i.e., the difference between nitrogen intake and fecal nitrogen output. However, for two reasons, this method is unsatisfactory for the precise estimation of the digestibility of individual amino acids. First, fecal nitrogen consists largely of bacterial protein, and because the composition of bacterial protein differs markedly from that of common dietary proteins, it gives very little information on the digestibility of different food-derived amino acids. Second, the bac-terial nitrogen is not only derived from undigested protein. This is because proteins secreted into the intestinal lumen, as well as the urea nitrogen that has diffused from the blood, are important contributors to colonic nitrogen flow. Studies in both animals and humans using 15N-labeled amino acids suggest that at least 50% of the fecal nitrogen is derived from the body rather than directly from undigested dietary protein.
Recently, 15N-labeled dietary proteins have been given to adults and by measuring the flow of 15N from the terminal ileum it is possible to calculate the “true” digestibility of the dietary source. There have also been a number of studies in pigs in which 15N-labeled amino acids have been infused intravenously for pro-longed periods. This labels the host proteins so that the 15N labeling of ileal proteins allows the calculation of the endogenous contribution of the luminal protein pool. By and large, the results of all these studies lead to the same conclusion; namely, that the true digest-ibility of most dietary proteins is very high and that at least 50% of the fecal nitrogen is derived from host metabolism rather than from the diet.
Most of the evidence favors the conclusion that there is an almost complete digestion of most dietary proteins in the small bowel. It is also quite clear that a considerable amount of amino acid metabolism occurs in the tissue of the splanchnic bed, in general, and in the intestinal mucosa, in particular, before the amino acids, liberated from food proteins during the digestive process, reach organs such as the liver, kidneys, and skeletal muscles. Calculations based on recent isotopic studies suggest that intestinal amino acid utilization (both from the diet and via the blood supply to the intestine; the mesenteric arterial circula-tion) can account for as much as 50% of the body’s utilization of amino acids. It is also important to note that the degree to which individual amino acids are utilized by the gut varies markedly (Table 4.11). Among the indispensable amino acids, threonine uti-lization is particularly high and virtually all of the dietary glutamate and aspartate are utilized within the mucosa. In addition, the magnitude of splanchnic amino acid metabolism varies with age, being appar-ently greater in infants and also perhaps in the elderly.
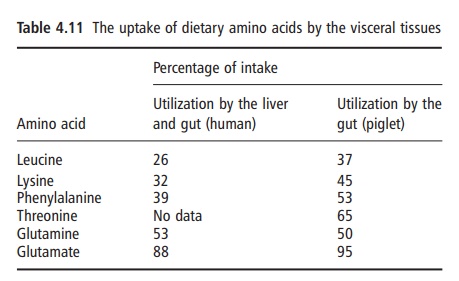
This can affect the efficiency with which the amino acids derived from the protein ingested are used to support overall body nitrogen and amino acid homeo-stasis and balance.
Protein nutritional quality
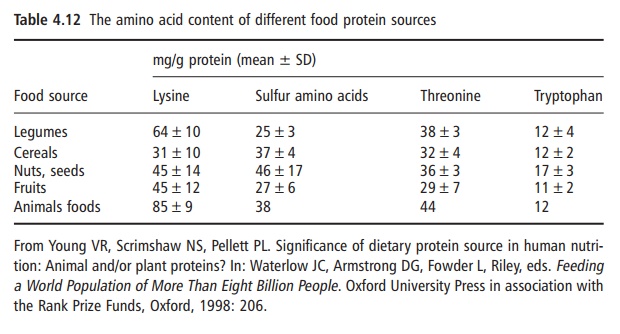
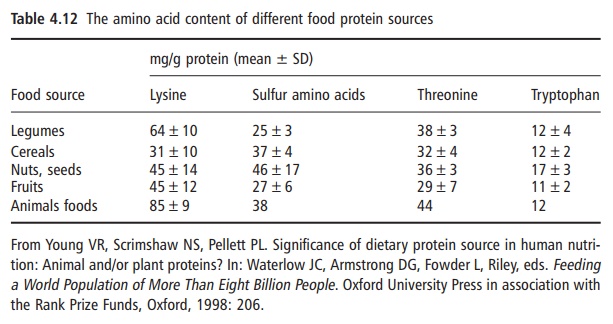
Not all proteins have the same capacity to meet the physiological requirements for total nitrogen and the indispensable amino acids. The concentration and availability of the individual indispensable amino acids are major factors responsible for the differences in the nutritive values of food proteins. Thus, the content and balance of indispensable amino acids differ among plant and animal protein foods. For the present purpose a summary is given in Table 4.12, listing the four indispensable amino acids that are most likely to be limiting, or in shortest supply and especially in food proteins of plant origin. As can be seen, lysine is present in a much lower concentration in all the major plant food groups than in animal protein foods and is most frequently the most
This can affect the efficiency with which the amino acids derived from the protein ingested are used to support overall body nitrogen and amino acid homeo-stasis and balance.
Protein nutritional quality
Not all proteins have the same capacity to meet the physiological requirements for total nitrogen and the indispensable amino acids. The concentration and availability of the individual indispensable amino acids are major factors responsible for the differences in the nutritive values of food proteins. Thus, the content and balance of indispensable amino acids differ among plant and animal protein foods. For the present purpose a summary is given in Table 4.12, listing the four indispensable amino acids that are most likely to be limiting, or in shortest supply and especially in food proteins of plant origin. As can be seen, lysine is present in a much lower concentration in all the major plant food groups than in animal protein foods and is most frequently the most limit ing amino acid.
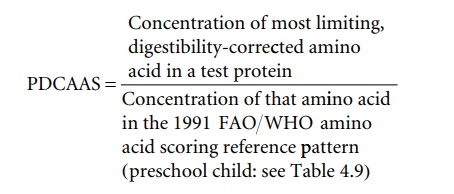
The nutritional signifi cance of these differences can be assessed in a number of ways. One useful approach is an amino acid scoring procedure that compares the content of amino acids in a protein with a reference human amino acid requirement pattern.
In 1991 a UN Expert Consultation reviewed the appropriate methods for measuring quality of food proteins for the nutrition of human populations. This consultation concluded that the most appropriate method available was the protein digestibilitycorrectedamino acid score (PDCAAS) method, and it was recommended for international use. This amino acid scoring procedure, including a correction for digestibility, uses the amino acid requirement pattern for a 2–5 year old child (as shown in Table 4.9). This is the reference amino acid requirement pattern for this purpose, expressing the amino acid requirement in relation to the total protein requirement.The PDCAAS is estimated from the following equation:
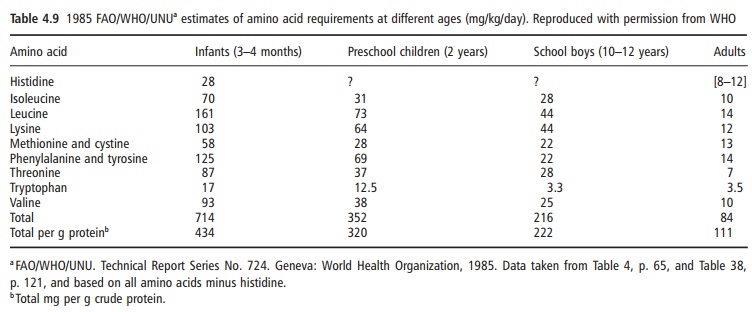
In addition to establishing the amino acid reference pattern for use in the PDCAAS method, the UN Consultation considered the procedures for measur-ing and estimating amino acids and digestibility. This approach offers considerable benefits over that of animal bioassays, which traditionally have been used to assess the quality of food protein in human diets. An important benefit is that the PDCAAS approach uses human amino acid requirements as the basis of evaluation, which ensures that appropriate levels of indispensable amino acids will be provided in the diet. In addition, use of the proposed amino acid scoring procedure facilitates an evaluation of blending of foods to optimize nitrogen utilization and meet
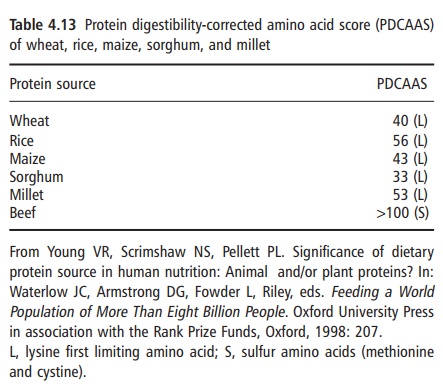
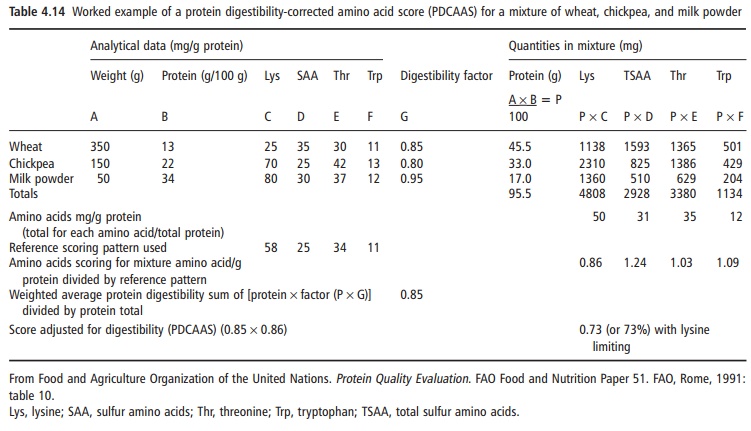
protein and amino acid needs. A listing of some calcu-lated PDCAAS values for selected food protein sources is given in Table 4.13 and a worked example for a mixture of food proteins is presented in Table 4.14.
The development of an internationally derived procedure for evaluating protein quality using the amino acid scoring concept is a step that had long been required. This PDCAAS procedure can be modi-fied as new knowledge about specific amino acid requirements emerges, as the determination of avail-ability of dietary amino acids is improved, and as the factors affecting digestibility and availability are better understood. For the present, the PDCAAS procedure would appear to be very useful for evaluating the nutritional quality of human food protein sources.
Major sources of food proteins in the diet
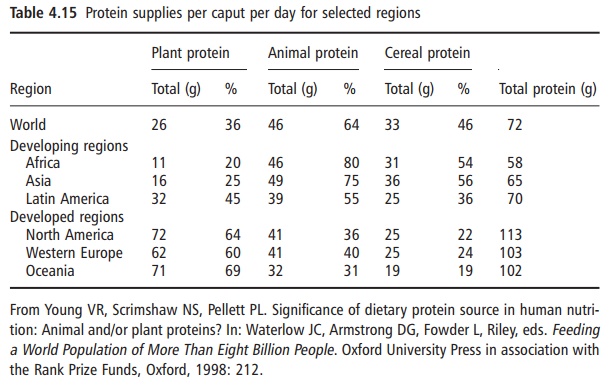
The relative proportions in the diet of food proteins of animal and plant origin differ according to geographi-cal region and other socioeconomic and cultural factors. Broadly, animal protein foods account for 60– 70% of the total protein intake in the developed regions (Table 4.15). In contrast, plant proteins make up about 60–80% of the total protein intake in developing regions, with cereals being the dominant source in this case. Given the differences in amino acid content of
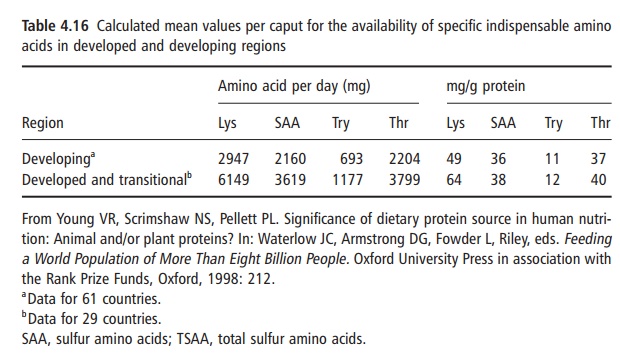
food proteins mentioned above it is not surprising that there are distinct differences in the intakes of the indis-pensable amino acids by different population groups worldwide. An example of such differences is given in Table 4.16. As already noted, the four amino acids of greatest importance and those most likely to be most limiting in intake, relative to requirements, are lysine, the sulfur amino acids (methionine and cystine), tryp-tophan, and threonine.
Related Topics