Chapter: Introduction to Human Nutrition: Nutrition and Metabolism of Proteins and Amino Acids
Biology of protein and amino acid requirements
Biology of protein and amino acid requirements
Body protein mass
A major and fundamental quantitative function of the dietary α-amino acid nitrogen and of the indis-pensable amino acids is to furnish substrate required for the support of organ protein synthesis and the maintenance of cell and organ protein content. Therefore, in the first instance the body protein mass is a factor that will influence the total daily require-ment for protein. Adult individuals of differing size but who are otherwise similar in age, body composi-tion, gender, and physiological state would be expected to require proportionately differing amounts of nitrogen and indispensable amino acids. Changes in the distribution and amount of body protein that occur during growth and development and later on during aging may be considered, therefore, as an initial approach for understanding the metabolic basis of the dietary protein and amino acid needs.
Direct measures of total body protein cannot yet be made in living subjects, although there are various indirect measures from which it is possible to obtain a picture of the body nitrogen (protein) content at various stages of life. From these approaches it is clear that body nitrogen increases rapidly from birth during childhood and early maturity, reaching a maximum by about the third decade. Thereafter, body nitrogen decreases gradually during the later years, with the decline occurring more rapidly in men than in women. A major contributor to this age-related erosion of body nitrogen is the skeletal musculature. Strength training during later life can attenuate or partially reverse this decline in the amount of protein in skeletal muscles and improve overall function.
The protein requirement of adults is usually considered to be the continuing dietary intake that is just sufficient to achieve a “maintenance” of body nitro-gen, often measured only over relatively short experimental periods. For infants and growing children and pregnant women an additional requirement is needed for protein deposition in tissues. However, this concept is oversimplified since the chemical com-position of the body is in a dynamic state and changes occur in the nitrogen content of individual tissues and organs in response to factors such as diet, hor-monal balance, activity patterns, and disease. Thus, proteins are being continually synthesized and degraded in an overall process referred to as turnover. The rate of turnover and the balance of synthesis and degradation of proteins, in addition to the mass of protein, are also important determinants of the requirements for nitrogen and amino acids, and these aspects will be discussed in the following section.
Turnover of proteins and amino acid metabolism
Protein synthesis, degradation, and turnover
The principal metabolic systems responsible for the maintenance of body protein and amino acid homeo-stasis are shown in Figure 4.5. They are:
●protein synthesis
●protein breakdown or degradation
●amino acid interconversions, transformation, and eventually oxidation, with elimination of carbon dioxide and urea production
●amino acid synthesis, in the case of the nutritionally dispensable or conditionally indispensable amino acids.
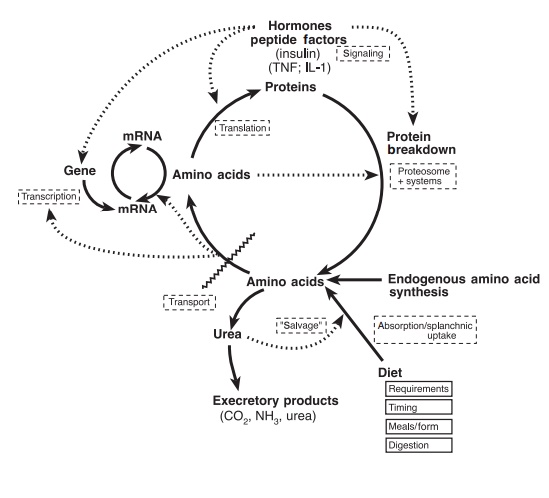
Figure 4.5 The major systems in amino acid uptake, utilization, and catabolism, with an indication of the processes involved and some factors that can affect them. TNF, tumor necro-sis factor, IL, interleukin.
Dietary and nutritional factors determine, in part, the dynamic status of these systems; such factors include the dietary intake levels relative to the host’s protein and amino acid requirements, the form and route of delivery of nutrients, i.e., parenteral (venous) and enteral (oral) nutritional support, and timing of intake during the day, especially in relation to the intake of the major energy-yielding substrates, which are the carbohydrates and fats in foods. Other factors, including hormones and immune system products, also regulate these systems. This will be a topic for discussion in the following volume. Changes in the rates and efficiencies of one or more of these systems lead to an adjustment in whole body nitrogen (protein) balance and retention, with the net direc-tion and the extent of the balance depending upon the sum of the interactions occurring among the pre-vailing factor(s).
In effect, there are two endogenous nitrogen cycles that determine the status of balance in body protein:
●the balance between intake and excretion
●the balance between protein synthesis and break-down (Figure 4.6).
In the adult these two cycles operate so that they are effectively in balance (nitrogen intake = nitrogen excretion and protein synthesis = protein break-down), but the intensity of the two cycles differs, the flow of nitrogen (and amino acids) being about three times greater for the protein synthesis/breakdown component than for nitrogen intake/excretion cycle.
Protein synthesis rates are high in the premature newborn, possibly about 11–14 g protein synthesized per kilogram of body weight per day, and these rates decline with growth and development so that in term babies and young adults these rates are about 7 g and
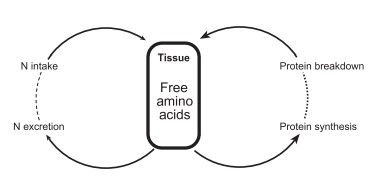
Figure 4.6 The two endogenous nitrogen cycles that determine the status of body protein (nitrogen) balance. (Adapted from Waterlow JC. The mysteries of nitrogen balance. Nutr Res Rev 1999; 12: 25–54, with permission of Cambridge University Press.)
4–5 g protein/kg per day, respectively. Three points relevant to nutritional requirements may be drawn from these data. First, the higher rate of protein syn-thesis in the very young, compared with that in the adult, is related not only to the fact that a net deposi-tion of protein occurs during growth, which may account for about 30% of the total amount of protein synthesized in the 6 month old infant, but also to a high rate of protein turnover (synthesis and break-down) associated with tissue remodeling and repair, as well as to removal of abnormal proteins. In the adult the protein turnover is associated with cell and organ protein maintenance since there is no net tissue growth under most circumstances. Second, as will be seen later, at all ages in healthy subjects the rates of whole body protein synthesis and breakdown are considerably greater than usual intakes (the latter are about 1–1.5 g protein/kg per day in adults) or those levels of dietary protein thought to be just necessary to meet the body’s needs for nitrogen and amino acids (about 0.8 g protein/kg per day). It follows, therefore, that there is an extensive reutilization within the body of the amino acids liberated during the course of protein breakdown. If this were not the case it might be predicted that we would be obligate carnivores and this, undoubtedly, would have changed the course of human evolution. Third, although not evident from this discussion alone, there is a general as well as functional relationship between the basal energy metabolism or resting metabolic rate and the rate of whole body protein turnover. Protein synthe-sis and protein degradation are energy-requiring pro-cesses, as will be described elsewhere in these volumes, and from various studies, including interspecies com-ponents, it can be estimated that about 15–20 kJ (4– 5 kcal) of basal energy expenditure is expended in association with the formation of each gram of new protein synthesis and turnover. In other words, protein and amino acid metabolism may be respon-sible for about 20% of total basal energy metabolism. Because basal metabolic rate accounts for a significant proportion of total daily energy expenditure, it should be clear from this discussion that there are significant, quantitative interrelationships between energy and protein metabolism and their nutritional require-ments. For these reasons it would not be difficult to appreciate that both the level of dietary protein and the level of dietary energy can influence the balance between rates of protein synthesis and protein break-down and so affect body nitrogen balance. Their effects are interdependent and their interactions can be complex. This can be illustrated by the changes in body nitrogen balance that occur for different protein and energy intakes (Figure 4.7); as seen here, the level of energy intake, whether above or below require-ments, determines the degree of change in the nitro-gen balance that occurs in response to a change in nitrogen intake. Conversely, the level of nitrogen intake determines the quantitative effect of energy intake on nitrogen balance. Therefore, optimum body protein nutrition is achieved when protein and
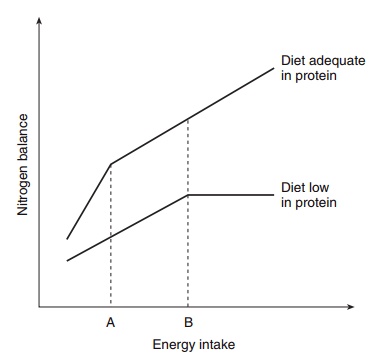
Figure 4.7 Relationship between nitrogen balance and energy intake with diets of different protein levels. Between energy intake A (low) and B (higher) the two lines are parallel. (Reproduced from Munro HN, Allison JB, eds. Mammalian Protein Metabolism, vol. I. New York: Academic Press, 1964: 381 with permission.)
energy intakes (from sources such as carbohydrates and lipids) are sufficient to meet or balance the needs for amino acids, nitrogen, and the daily energy expen-diture or, in the case of growth, the additional energy deposited in new tissues.
Amino acids as precursors of physiologically important nitrogen compounds
As already pointed out, amino acids are also used for the synthesis of important nitrogen-containing com-pounds that, in turn, play critical roles in cell, organ, and system function. In carrying out these particular roles the amino acid-derived metabolites also turn over and they need to be replaced ultimately by the nitrogen and indispensable amino acids supplied by protein intake. Estimates on the quantitative utiliza-tion of these precursor and nonproteinogenic roles of amino acids in human subjects are limited but it is possible to give some examples.
●Arginine is the precursor of nitric oxide (NO); the total amount of NO synthesized (and degraded) per day represents less than 1% of whole body arginine flux and less than 1% of the daily arginine intake.
●In contrast, the rate of synthesis and degradation of creatinine is relatively high and accounts for 10% of the whole body flux of arginine and for 70% of the daily intake of arginine.
Similarly, the synthesis and turnover of glutathione (a major intracellular thiol and important antioxi-dant, formed from glutamate, glycine, and cyste-ine) accounts for a high rate of cysteine utilization such that it greatly exceeds the equivalent of the usual daily intake of cysteine. Since continued glu-tathione synthesis involves a reutilization of endog-enous cysteine, a low intake of dietary methionine and cyst(e)ine would be expected to have an unfa-vorable influence on glutathione status and synthe-sis. This has been shown experimentally to be the case, especially in trauma patients and those suffer-ing from acquired immunodeficiency syndrome (AIDS). Because glutathione is the most important intracellular antioxidant that protects cells against damage by reactive oxygen species, this would mean that particular attention should be paid to such amino acids in nutritional therapy in these groups of patients.
Urea cycle enzymes and urea production
Finally, with reference to the major processes shown in Figure 4.5, the urea cycle enzymes, which are dis-tributed both within the mitochondrion and in the cytosol (Figure 4.8), are of importance. The produc-tion of urea may be viewed largely, but not entirely, as a pathway involved in the removal of amino nitro-gen and contributing to an adjustment of nitrogen loss to nitrogen intake under various conditions. The five enzymes of urea biosynthesis associate as a tightly connected metabolic pathway, called a metabalon, for conversion of potentially toxic ammonia as well as removal of excess amino acids via their oxidation with transfer of the nitrogen to arginine and ulti-mately urea. This is especially important when the supply of protein or amino acids is high owing to
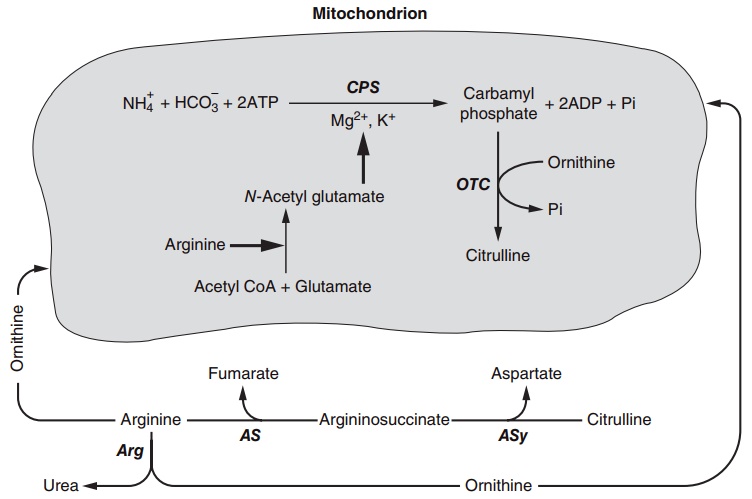
Figure 4.8 The urea cycle enzymes and their distribution in the liver. CPS, carbamoyl phosphate synthetase; OTC, ornithine transcarbamylase; Asy, argininosuccinic synthetase; AS, argininosuccinate; Arg, arginase.
variations in the exogenous intake or when there is a high rate of whole body protein breakdown in cata-bolic states, as occurs in severe trauma and following overwhelming infection.
The reutilization of urea nitrogen starts from the hydrolysis of the intact urea molecule. By constantly infusing the [15N2]-urea tracer, the appearance of the singly labeled [15N]-urea should represent the extent of urea hydrolysis. A 24 hour constant infusion of [15N2]-urea revealed a minimal amount of [15N]-urea appearance in the plasma, and a linear relationship over a wide range of protein intake versus total urea production and urea hydrolysis. Furthermore, the possible metabolic pathways involved in the assimila- tion of ammonia generated from urea nitrogen include (1) citrulline synthesis, (2) L-glutamate dehy-drogenase pathway in the mitochondria, and (3) glycine synthase. The net formation of amino nitro-gen from these pathways is quantitatively minimal compared with the metabolic fluxes of these amino acids through their major pathways, such as protein turnover, dietary intake, and de novo synthesis (of the nutritionally dispensable amino acids only).
Summary of the metabolic basis for protein and amino acid requirements
It should be evident from this account of the underly-ing aspects of the needs for α-amino nitrogen and indispensable amino acids, that the “metabolic” requirement can usefully be divided: first, into those needs directly associated with protein deposition, a critical issue in infants, early childhood nutrition, and during recovery from prior depletion due to disease or malnutrition; and, second, into those needs associ-ated with the maintenance of body protein balance, which accounts for almost all of the amino acid requirement in the healthy adult, except for that due to the turnover and loss of the various physiologi-cally important nitrogen-containing products, some of which were mentioned above. Quantifying the minimum needs for nitrogen and for indispensable amino acids to support growth should be relatively easy, in principle, because these needs are simply the product of the rate of protein nitrogen deposition and the amino acid composition of the proteins that are deposited. Here, it may be pointed out that the gross amino acid composition of whole body proteins shows essentially no difference among a variety of mammals, including humans (Table 4.6). Thus, at the major biochemical level the qualitative pattern of the needs of individual amino acids to support protein deposition would be expected to be generally similar.
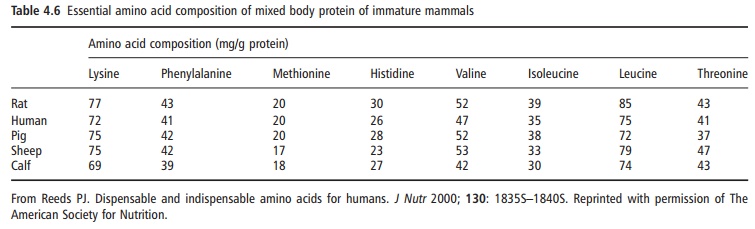
In humans, in contrast to rapidly growing mammals such as the rat and pig, the obligatory amino acid needs for the purposes of net protein deposition are for most stages in life a relatively minor portion of the total amino acid requirement. Hence, most of the requirement for nitrogen and amino acids is associ-ated with the maintenance of body protein stores (or body nitrogen equilibrium). A major portion of the maintenance nitrogen and amino acids needs is directly associated with protein metabolism and reflects two related factors.
●Amino acids released from tissue protein degrada-tion are not recycled with 100% efficiency.
●Amino acid catabolism is a close function of the free amino acid concentration in tissues, and so the presence of the finite concentrations of free amino acids necessary to promote protein synthesis inevi-tably leads to some degree of amino acid catabolism and irreversible loss.
The other metabolic component of the require-ment for nitrogen and amino acids, as mentioned above, is due to the turnover of functionally impor-tant products of amino acid metabolism, which are also necessary to maintain health. Although this may not necessarily be a major quantitative component of the daily requirement, it is qualitatively and function-ally of considerable importance; health depends on the maintenance of this component of the protein need.
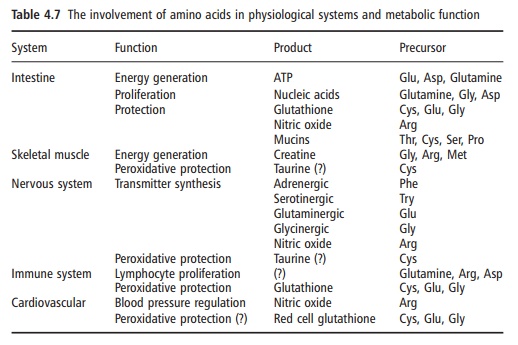
Finally, four physiological systems appear to be critical for health: the intestine, to maintain absorptive and protective function; the immune and repair system and other aspects of defense; the skeletal musculature system; and the central nervous system. Within each system it is possible to identify critical metabolic roles for some specific amino acids (Table 4.7). Also of note is that, with certain exceptions (the involvement of phenylalanine and tryptophan in the maintenance of the adrenergic and serotinergic neu-rotransmitter systems, and methionine as a methyl group donor for the synthesis of creatine, as well as the branched-chain amino acids as nitrogen precur-sors for cerebral glutamate synthesis), the necessary precursors shown here are the dispensable and con-ditionally indispensable amino acids.
Related Topics