Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Sensory systems
Mechanoreception - Fishes
Mechanoreception
Water’s density makes it an excellent conductor of vibrations. It is not surprising, therefore, that aquatic organisms have come to rely heavily on detecting these signals in a variety of ways. These mechanisms evolved early in the long history of vertebrates, and have become highly modify ed and specialized in the fishes.
Mechanoreception among fishes involves the detectionof the movement of the water. Fishes have two majormechanosensory systems: the lateral line system and theinner ear. Both of these rely on sensory hair cells (Fig. 6.1)which include an array of cilia on their apical surface. Displacementof the shorter stereo cilia with respect to themuch longerKino cilium alters the rate of nerve impulses sent to the brain by the nerve cells associated with each hair cell – a higher rate if the stereo cilia move toward the Kino ciliumand a lower rate if they move in the opposite direction.The lateral line system detects disturbances in the water, thereby helping a fish detect currents, capture prey,maintain position in a school, and avoid obstacles and predators, whereas the inner ear is responsible for fish equilibrium and balance, as well as hearing (Schell art&
Wubbels 1998).
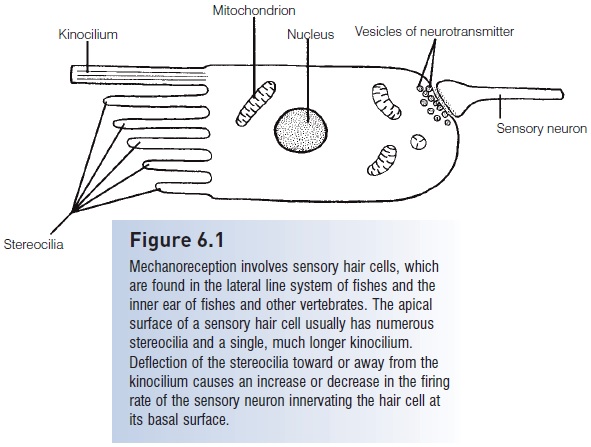
Figure 6.1
Mechanoreception involves sensory hair cells, which are found in the lateral line system of fishes and the inner ear of fishes and other vertebrates. The apical surface of a sensory hair cell usually has numerousstereocilia and a single, much longer Kino cilium.Deflection of the stereo cilia toward or away from the Kino cilium causes an increase or decrease in the firing rate of the sensory neuron innervating the hair cell atits basal surface.
Lateral line system
The lateral line system is an old feature in the history ofvertebrates, as indicated by its presence in fossil jawlessfishes from the Silurian. The system is only useful in water,and is therefore restricted to fishes and larval and permanently aquatic amphibians. The hair cells of the lateral linesystem are organized into neuromasts, which allow fishesto detect vibrations in the water that originate from orreflect off prey, predators, other fishes in a school, andenvironmental obstacles. These neuromasts are clusters ofcells that are typically covered by a gelatinous cupula (Fig.6.2), which can be displaced by water movement, there by moving the cilia of the hair cells and initiating a change
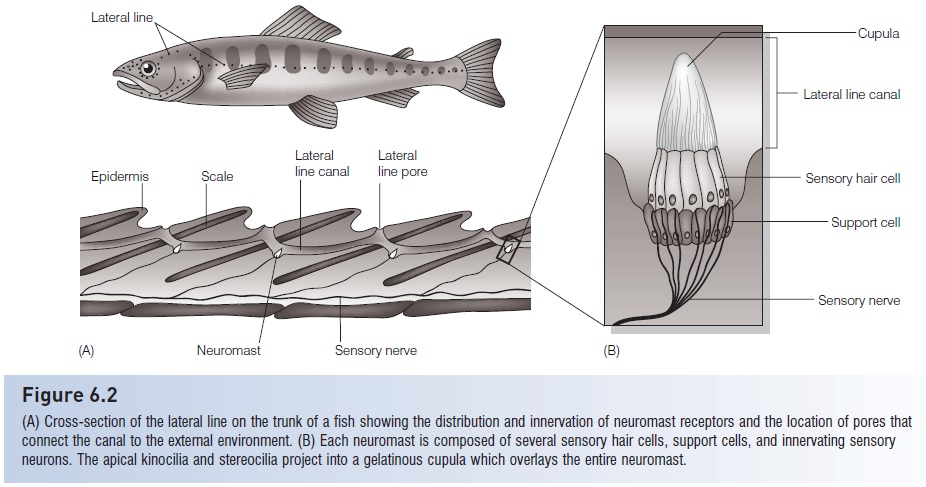
Figure 6.2
(A) Cross-section of the lateral line on the trunk of a fish showing the distribution and innervation of neuromast receptors and the location of pores thatconnect the canal to the external environment. (B) Each neuromast is composed of several sensory hair cells, support cells, and innervating sensory neurons. The apical Kino cilia and stereo cilia project into a gelatinous cupula which overlays the entire neuromast.
The lateral line system has two main subdivisions –superficial neuromasts, which are free-standing on the skin, and canal neuromasts, which are located in channelsbeneath the scales of the trunk (the “lateral line”) and in dermal bones of the head (“cephalic lateral line canals”)and which open to the surrounding water via small pores(Fig. 6.2). Early in development all neuromasts are superficial and tend to be concentrated around the head, but asdevelopment progresses they spread along the trunk andin many fishes they become incorporated into canals thatrun below the skin or scales (Poling & Fuiman 1998; Diazet al. 2003; Gibbs & Northcutt 2004). Superficial neuromastsare more exposed, making them quite sensitive towater movement across the skin. This makes them particularly effective for detecting water currents fororientation(rheotaxis) or movement of the fish itself in areas with littlewater velocity, but not very useful for detecting smallstimuli in areas of swift or turbulent water (Engelmannet al. 2000, 2002). They also are most effective in detecting currents that are unidirectional or at frequencies below20 Hz (Braun et al. 2002). Superficial neuromasts are moreabundant in fishes that are sedentary or slow swimmers andthat inhabit quiet areas, such as the Goldfish (Carassiusauratus). Canalneuromasts, however, are shielded from constant stimulation by water moving across the skin andare better at detecting stimuli if the fish or the water around it is moving quickly. Therefore, they are more effective in detecting transient currents, or currents of higher frequency(20–100 Hz; see Braun et al. 2002). These, therefore, tendto be better developed in fishes that are fast swimmers or that live in fast or turbulent water. Rainbow Trout(Oncorhyunchus mykiss), for example, often inhabitrunning water and have very few superficial neuromastsbut have well-developed neuromasts in narrow canals(Engelmann et al. 2002). And the canal neuromasts of theMottled Sculpin (Cottus bairdi) help the fish locate prey byfiltering out background stimuli due to water currents(Kanter & Coombs 2003).
As an example of the relationship between the lateral line system and habitat use, Poling and Fuiman (1998)studied the development of lateral line systems and visionin juveniles of three species of drum (family Sciaenidae). Asin other fishes, the superficial neuromasts develop first, andthen some become incorporated into canals as developmentcontinues and the fishes become more active. However, the relative abundance of the two types of neuromasts differed among species and correlated well with juvenile habitat and the relative role that vision might also play. Juvenilesof Spotted Seatrout (Cynoscion nebulosus), which inhabit shallow, murky, and often weedy inshore areas wheremechanoreception would be more critical to predator andprey detection, had significantly more superficial neuromastson their heads than did juveniles of Red Drum(Sciaenops ocellatus) or Atlantic Croaker (Micropogonisundulatus). Juveniles of Atlantic Croaker settle the furthest offshore, where the water is clearer and deeper, and they are larger and have the best developed eyes of the three species. Red Drum juveniles are somewhat intermediate in both their habitat (bays and nearshore areas) and their sensory development.
There are other examples of fishes in which mechanoreceptionhelps compensate for a poor visual environment.
Under experimental conditions, Lake Trout (Salvelinusnamaycush) detected and followed the hydrodynamic trails left by prey fishes in total darkness, and their ability tocapture prey was significantly inhibited when the lateral line system was rendered ineffective (Montgomery et al.2002). Mottled Sculpin feed in low light conditions andrely on their lateral lines to detect and locate prey (Braunet al. 2002). And Blind Cave Fish (Astyanax fasciatusmexicanus/Anoptichthys jordani) have many superficialneuromasts, as well as taste buds, on their heads to helpcompensate for the inability to see (Schell art& Wubbells1998). They rely more on their lateral line system than anyother sense (Montgomery et al. 2001a).
Some fish predators have learned that their prey canbe attracted to vibrations and have used this to theiradvantage. Several species of piscivorous birds, includingherons and egrets (family Ardeidae) have been observedcreating disturbances in the water’s surface by tongue flicking or bill-vibrating in order to attract fishes (Davis2004). In addition, the recreational angling industrydesigns lures that create vibrations in the water, and evenfishing rods have been developed that have built-in,battery-operated vibration devices that claim to enhance angling success.
Equilibrium and balance
Hair cells also can detect movement of fluid or objects within organisms, and therefore play an important role in the ability of fishes to maintain their equilibrium and orientation within the water column (Schell art& Wubbels1998). Postural equilibrium and balance are maintained by the pars superior, a portion of a fish’s inner ear that, in jawed fishes, consists of three semicircular canals and an additional chamber known as the utricle (Fig. 6.3).Lampreys (Petromyzontidae) have only two semicircular canals, and hagfishes (Myxinidae) have one. The semicircular canals are filled with a fluid (endolymph) and have sensory hair cells in their terminal ampullae. Changes in acceleration or orientation set the endolymph in motion and cause displacement of a gelatinous cupula that encloses the cilia of the hair cells. Lateral displacement of the cilia results in changes in the fi ring rate of the sensory neurons innervating the hair cells, thereby signaling the fish’s brain about changes in acceleration or orientation. The utricle contains a solid deposit, or otolith (“ear stone”), the lapillus, which rests on a bed of sensory hair cells. The downward pull of gravity on the lapillus triggers impulses from the sensory cells and provides the fish with information regarding its vertical orientation in the water. The utricle works in coordination with the detection of light from above the fish by the retina of the eyes and together they help keep the fish upright in the water (the dorsal lightreflex). Goldfish with the utricle and semicircular canals removed on one side initially lost their ability to orient vertically, although this was often regained after several days (Ott & Platt 1988).
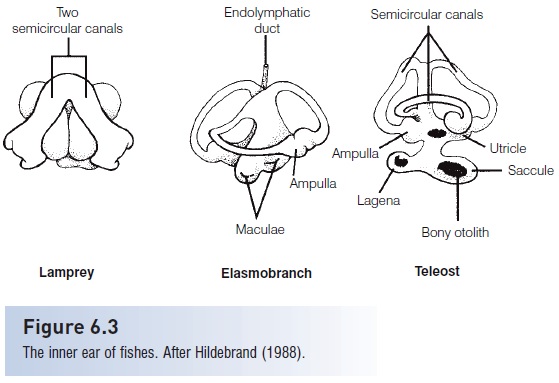
Figure 6.3
The inner ear of fishes.After Hildebrand (1988).
Hearing
Hearing in fishes is primarily the responsibility of the inner ear, including the utricle of the pars superior and the sacculeandlagenaof the pars inferior (see Fig. 6.3). Each chamber contains an otolith (the lapillus, sagitta and astericus,respectively) and is lined with patches of tissue composed of sensory hair cells. An otolithic membrane provides a mechanical linkage between the otolith and the cilia of the sensory hair cells.
Most fish tissue is transparent to sound because its density is similar to that of the water. Structures that are significantly different in density, however, will vibrate differently from the rest of the fish’s tissues and provide an opportunity for sensory detection of sound. As sound vibrations pass through a fish, the otoliths lag behind in their vibration due to their greater density. The relative difference in vibration between the fish’s sensory hair cells and the otoliths excites the sensory hair cells and triggers action potentials in the sensory neurons of the auditory nerve(Schell art& Wubbels 1998).
Aquatic environments are quite varied in the levels of background sound, and not surprisingly hearing sensitivity of fishes is well matched to their habitats. Fishes in noisy habitats, such as coastlines and swift rivers and streams, tend to have higher sound thresholds and narrower ranges for sound detection than fishes in calmer, quieter habitats such as small lakes and ponds with soft substrate bottoms(Schell art& Wubbels 1998). Sound waves may cause gas spaces in a fish, such as a gas bladder, to vibrate, thereby providing an opportunity to enhance sound detection. Fishes that are hearing specialists have mechanisms that transmit gas bladder vibrations to the inner ear for detection by the otolith organs, whereas fishes without such a connection, or that lack a gas bladder, are not as sensitive to sound and are referred to as hearing generalists (Yan2003). Hearing specialists include the cods (Gadidae),which have a gas bladder close to the inner ear, and squirrelfish (Holocentridae) and herring and sardines (Clupeidae)in which anterior extensions from the gas bladder contact the inner ear (Akamatsu et al. 2003). Africanmormyrids (Mormyridae), also known for sensitive hearing, have separate otic gas bladders adjacent to the inner ear that assist with sound detection (Yan &Curt singer 2000).And the saccule of the inner ear of gourami’s (Anabantoidei)protrudes into the upper part of the air-filled suprabranchialchamber (used for air breathing), thereby significantly enhancing the fishes’ hearing sensitivity (Yan 1998).
The largest group of hearing specialists is the otophysanfishes, which dominate the fresh waters of the world (over60% of all freshwater species). They have particularly acute hearing and pitch discrimination due to the Weberian ossicles,a series of small bones derived from modified vertebrae that connect the anterior end of the gas bladder to the inner ear (Fig. 6.4). These conduct sound vibrations in
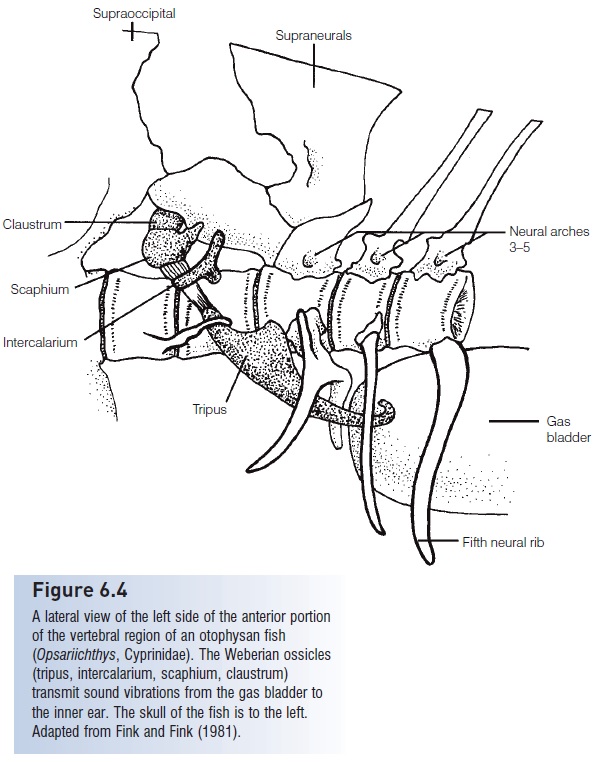
Figure 6.4
A lateral view of the left side of the anterior portion of the vertebral region of an otophysan fish(Opsariichthys, Cyprinidae). The Weberian ossicles(tripus, intercalarium, scaphium, claustrum)transmit sound vibrations from the gas bladder to the inner ear. The skull of the fish is to the left. Adapted from Fink and Fink (1981).
Because of the Weberian ossicles and gas bladder, the otophysans have the highest sensitivity and greatest frequency range of hearing among fishes. Interference with the Weberian ossicles of the otophysans, or deflation of the gas bladder of any of the hearing specialist fishes, results in decreased hearing sensitivity (Yan et al. 2000).
Much less is known about hearing in elasmobranchs than in bony fishes. The basic structure of elasmobranch inner ears is very similar to that of bony fishes, except that sharks have a connection from a depression in the back of their skulls to ducts in the semicircular canals that contain the sensory area (Hueter et al. 2004). This connection,called the fenstra ovalis, is presumed to enhance hearing by directing sound to the sensory area (macula neglecta) of the inner ear. Shark hearing is most sensitive at low frequencies, including those below 10 Hz, which are undetectable by humans, but they may be no more sensitive than many other fishes that are hearing generalists. Sharks are most sensitive to pulsed sounds in this range, such as those emitted by the erratic swimming of an injured fish, and can localize such sounds at distances of up to 250 m (Myrberg1978; Myrberg & Nelson 1991; Subclass Elasmobranchii, Sensory physiology).
Sources of underwater sound
Apparently, natural selection pressures have driven the evolution of various adaptations to enhance hearing among fishes. So it must be important for some fishes to hear well– but what do they hear that could be so important? OneSource of sounds is predators. Cetaceans, such as dolphins, emit sounds and use echolocation for orientation and to locate prey. Although most marine fishes studied thus far cannot detect sound frequencies above about 500 Hz,at least some clupeids can detect considerably higher frequencies. The ability of some clupeids to detect ultrasound has been attributed to the anterior extensions of the gas bladder that is characteristic of the group. However, not all clupeids can detect ultrasound, and Higgs et al. (2004)believe that modifications of the utricle seen in those clupeids that can detect ultrasound is responsible for thisability. The American Shad (Alosa sapidissima), Alewife(A. pseudoharengus), and Pacific Herring (Clupea pallasii)can detect ultrasonic signals (up to 180 kHz in American Shad), and may use this ability to avoid predatory cetaceans trying to locate them using ultrasound (Plachta &Popper 2003). Another clupeid, the Spot linedSardine(Sardinops melanostictus), can detect sounds of 1 kHz(Akamatsu et al. 2003).
Another source of underwater sounds are fishes themselves– many of which create sounds for inter- and intraspecific communication. Fishes can produce sounds using several different mechanisms. Some catfishes (Siluriformes),toadfishes (Batrachoididae), cods (Gadidae), and sea robins(Triglidae) use muscles to create sound from the gas bladder(see Ladich & Yan 1998). Some catfishes rub together bones of the pectoral girdle, whereas some cichlids and gouramis(Anabantoidei) create sound by grinding together their pharyngeal teeth. The croaking gouramis (Trichopsis) create pulsed sounds by strumming tendons over elevations of fin rays by rapidly beating their pectoral fins. The close proximity of the anabantoid sonic organs to the suprabranchial chamber, which is used for air breathing, suggests that this air-filled chamber may enhance the resonance of the sound produced. The close relationship between the frequencies of vocalization and maximum hearing sensitivity also suggests that these features coevolved (Ladich & Yan 1998).
Fishes detect ambient sounds from their environment, and alter their behavior accordingly. Biological sound, suchas that produced by other fishes and invertebrates, attracts larval reef fishes to preferentially settle in areas with sounds that would indicate a suitable habitat (Montgomery et al.2001b). Ambient noise may also impact the evolution of hearing and sound production for communication. Males of two species of freshwater gobies (Gobiidae) that inhabit swift, rocky streams respond to and produce courtship sounds at frequencies within a “quiet window” of ambientnoise around 100 Hz (Lugli et al. 2003). The hearing of these fishes is most sensitive within this range, suggesting that ambient sound may be a selective force in the evolution of hearing and noise production.
The sensitivity of fishes, especially hearing specialists, to sound makes them potentially vulnerable to humangenerated underwater noise. McCauley et al. (2003) showed that high-intensity, low-frequency sound produced by airguns used in marine petroleum exploration can cause severe damage to the hair cells of fish inner epithelia. And even less intense sound can result in loss of hearing sensitivity in the otophysan Fathead Minnow (Pimephales notatus),although the less sensitive hearing generalist Bluegill(Lepomis macrochirus) was not significantly affected(Scholik & Yan 2002). Prolonged exposure of Goldfish(Carrasius auratus) to loud sound resulted in hearing loss in this otophysan species (Smith et al. 2004).
Related Topics