Chapter: Essentials of Psychiatry: Cognitive Neuroscience and Neuropsychology
Major Subdivisions of Memory Systems
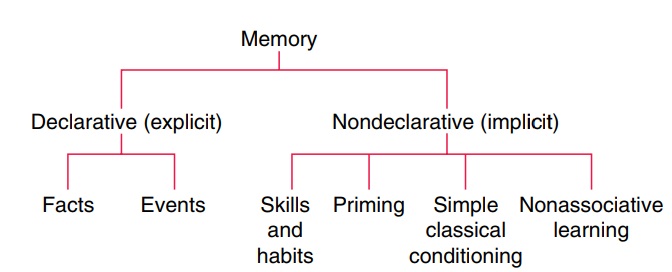
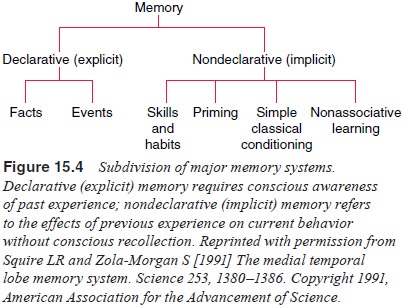
Major Subdivisions of Memory
Systems
Current research on the cognitive neuroscience of
memory has considered memory from the perspective of multiple systems rather
than a unitary system. Figure 15.4 illustrates the subdi-visions between memory
systems. A number of systems have been proposed, and the distinction between
these systems is not impermeable. The most basic distinction between memory
sys-tems is that of explicit versus implicit memory. Explicit memory is also
referred to as declarative memory and implicit memory is also known as
nondeclarative memory. What is considered explicit about this type of memory is
that it requires conscious awareness of past experience (Cohen and Squire,
1980). There are two major components of explicit memory: episodic memory and
semantic memory. Episodic or autobiographical memory is the
ability to remember personal events over time. This
refers to the individual’s ability to remember not only that something occurred
but also the context in which it occurred. Semantic memory, in contrast, refers
to knowledge without context. Another type of explicit memory system that
involves the short-term registering of information is termed working memory.
Memory systems research in the past several years has also focused on implicit
memory. Implicit memory does not involve conscious awareness. It refers to the
effects of previous experience on cur-rent behavior without conscious
recollection.
On the basis of cases of medial temporal lobe
damage in humans, it has been concluded that the medial temporal lobe,
including the hippocampus, and adjacent anatomical structures enable the
formation of explicit memories. In humans, it has been shown that a lesion
confined solely to the hippocampus (field CA1 of the hippocampus)
can produce a mild amnestic syndrome (Zola-Morgan et al., 1986). It would then appear that the para-hippocampal and
perirhinal cortical regions are also necessar-ily involved in explicit memory.
According to Zola-Morgan and Squire (1993), the medial temporal lobe system
essentially coor-dinates the organization of information that originated in
other brain regions. The medial temporal lobe system may thereby act as a
temporary site to store information that is cortically distrib-uted until the
information is permanently coded.
Diencephalon and Explicit Memory
Neuropsychological studies of patients with Wernicke–Korsakoff disease have yielded insight into the contribution of the dien-cephalon (dorsal and anterior thalamic nuclei and the mamillary bodies of the hypothalamus) to memory. Wernicke–Korsakoff disease is usually associated with a thiamine deficiency and is typically seen in individuals with alcoholism, nutritional defi-ciencies, infections and brain tumors (Markowitsch and Pritzel, 1985). The memory deficit is characterized by impairment of ex-plicit memory as seen by dense anterograde amnesia and variable retrograde amnesia. These patients are also frequently apathetic and indifferent and have diminished initiative.
The effects of damage caused by alcoholic and
nonalco-holic lesions to the anterior and medial thalamic nuclei as well as to
the mamillary bodies are diverse. The diencephalic region is connected with the
hippocampal region; the mamillary bodies are connected to the hippocampus via
the fornix and to the an-terior thalamus via the mamillothalamic tract. The
mediodorsal thalamic component of the system is also interconnected with the
frontal lobes. Patients with Korsakoff’s disease typically mani-fest frontal
lobe damage, which is seen in the phenomenon of confabulation. Aside from this
frontal component, which is often seen in alcoholism and may represent an
independent lesion, the diencephalic amnesia is largely similar to the kinds of
memory deficits observed with damage to the medial temporal lobe sys-tem. This
suggests that, at least at a functional level, anatomical damage in this
subcortical system produces an impairment in the consolidation of information.
Basal Forebrain and Memory
Another neuroanatomical region that plays a direct
role in ex-plicit memory is the basal forebrain. The basal forebrain region is
located where the diencephalon meets the cerebral hemispheres and includes a
number of brain structures such as the septal area, diagonal band of Broca,
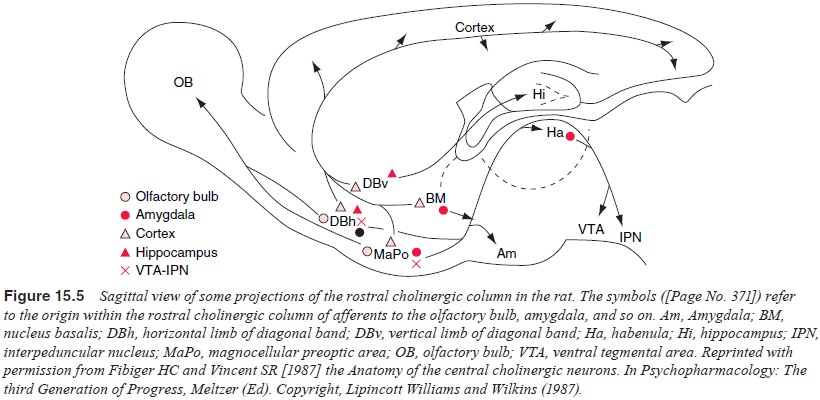
nucleus accumbens septi, olfactory tu-bercle, and substantia innominata. Figure
15.5 illustrates the ros-tral cholinergic projections in the rat brain. The
contribution of this brain region to memory has been a relatively recent
discov-ery. The cholinergic system plays a role in memory (although its full
role is not clear at this time), and is implicated in normal and pathological
aging, but is only one locus in the network of brain regions involved in
memory.
Affective Valence and Neuromodulatory Systems
Memory functioning requires a system for establishing va-lence between memorable events. That is, some events are more memorable than others. Affect and its associated chemical neuromodulators probably serve a valence capacity by facilitating the storage of emotionally charged experiences. The underlying assumption here is that emotionally tinged experiences activate neurobiological pathways that facilitate their storage. In evolu-tionary terms, it is highly adaptive to remember experiences that are learned under arousing conditions. Neuroimaging studies have provided evidence for the role of the amygdala in forming declarative memories. A number of neuromodulators play a role in affective responding and also influence memory storage. Chief among them is the noradrenergic system. The central noradrener-gic system involves the locus coeruleus in the midbrain reticular formation, the amygdala and the stria terminalis (a major afferent and efferent pathway to the amygdala). A number of findings have converged to suggest that central norepinephrine (NE) receptors in the amygdala are involved in the postlearning consolidation of information (Liang et al., 1986).
Overall, the role of the noradrenergic and other
systems, such as GABA and opioid peptides, in memory may lie in the re-lease of
adrenal epinephrine after stressful or emotional stimuli. Activation of NE in
the locus coeruleus–amygdala system, which ultimately has extensive
connectivity with a number of brain re-gions, then serves to consolidate the
storage of these memories. On a cellular level this may occur by the production
of LTP, be-cause noradrenergic compounds have been found to enhance LTP (Gold et al., 1984).
Related Topics