Chapter: Engineering Chemistry: Surface Chemistry and Catalysis
Langmuir Adsortion Isotherm
Langmuir
Adsortion Isotherm
Adsorption
Isotherm : One of the drawbacks of the Freundlich adsorption
isotherm is that it fails at high
pressure of the gas. Langmuir derived an adsorption isotherm on theoretical
considerations based on kinetic theory of gases. This is named as the Langmuir
adosrption isotherm. This isotherm is based on the assumption that every
adsorption site is equivalent and that the ability of a particle to bind there
is independent of whether or not nearby sites are occupied. In his derivation,
Langmuir considered adsorption to consist of the following two opposing
processes :
Asorption of the gas molecules on the surface of
the solid.
Desorption of the adsorbed molecules from the
surface of the solid.
Langmuir believed that eventually a dynamic
equilibrium is established between the above two opposing processes. He also
assumed that the layer of the adsorbed gas is only one molecule thick i.e.,
unimolecular. Since such type of adsorption is obtained in the case of chemisorption. Langmuir adsorption
isotherm works particularly well for chemisorption.
The Langmuir adsorption isotherm is represented by
the relation.

where a
and b are two Langmuir parameters. At
very high pressure, the above isotherm acquires the limiting form.
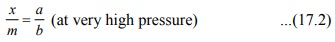
At very low pressure, Eq. (17.1) is reduced to x/m = ap (at very low pressure)
.... (17.3)
In order to determine the parameters a and b, Eq.
(17.1) may be written in its inverse form:
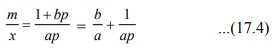
A plot of m/x
against 1/p gives a straight line the
slope and intercept equal to 1/a and b/a, respectively. Thus, both parameters
can be determined.
The Langmuir isotherm, in the form of Eq. (17.1) is
generally more successful in interpreting the data than the Freundlich isotherm
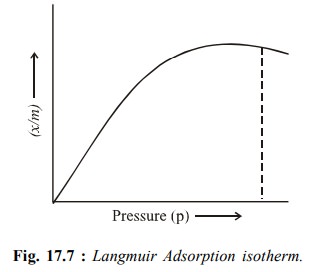
when a monolayer is formed. A plot of x/m
versus p is shown in (Fig17.7). At
low pressures, according to Eq. (17.3), pressure x/m increases linearly with p.
At high pressure according to Eq. (17.2), x/m becomes constant i.e. the surface
is fully covered and change in pressure has no effect and no further adsorption
takes place, as is evident from Fig. 17.7.
Adsorption from Solutions.
Adsorption occurs from solutions also. The solute
gets adsorbed on the surface of a solid adsorbent. Charcoal, a good adsorbent,
is often used to adsorb acetic acid, oxalic acid and organic dyestuffs from
their aqueous solutions.
The extent of adsorption, x/m depends upon the
concentration c of the solute. Freundlich isotherm is applicable to adsorption
from solutions when concentration is used in place of pressure as shown below.
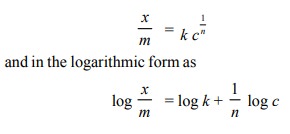
The plot of log
x/magainst c is also a
straight line, provided very low and very high concentrations are avoided.
Related Topics