Chapter: Engineering Chemistry: Surface Chemistry and Catalysis
Factors Affecting Adsorption
Factors
Affecting Adsorption
Adsorption occurs on the surface of almost all
solids. However, the extent of adsorption of a gas on the surface of a solid
depends upon the following factors :
(i) Nature and surface area of the adsorbent
(ii) Nature of
the adsorbed gas
(iii) Temperature
(iv) Pressure
of the gas
Let us now discuss these factors briefly.
(i) Nature and Surface Area of the Adsorbent
Different solids would adsorb different amounts of
the same gas even under similar conditions. Substances like charcoal and silica
gel are excellent adsorbents. The substances that are porous in nature and have
rough surfaces are better adsorbents.
The extent of adsorption also depends upon the surface
area of the solid. Greater the surface area, more is the surface available for
adsorption and greater is the adsorption. The surface area depends upon the
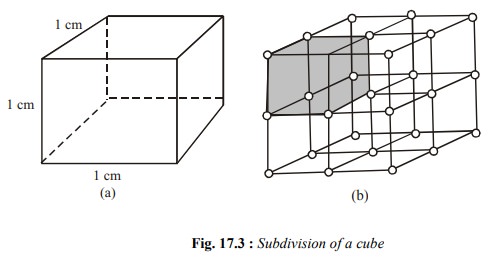
particle size of the substance. A cube of each side equal to 1cm has six faces.
Each of them is a square with surface area of 1cm2. Thus, the total
surface area of this cube is 6 cm2 Fig. 17.3 (a). If its each side
is divided into two equal halves, ½ cm long, and the cube is divided into two
equal halves, ½ cm long, and the cube is cut along the lines indicated in the
Fig (b), the cube would be divided into 8 smaller cubes with each side 0.5 cm
long [Fig. 17.3 (b)]. Surface area of each small cube would be (6 ´ 0.5 ´ 0.5) = 1.5 cm2 and
the total surface area of all the 8 smaller cubes would be 12 cm2
which is double the surface area of the original cube. If it is subdivided into
smaller cubes, each of side equal to 1 ´ 10–6
cm the surface area will increase to 6 ´ 106
cm2 or 600 m2. The increase in surface area would result
in greater adsorption.
Now we can explain why the solids that are porous
in nature and have rough surfaces are better adsorbents. It is so because each
of these features increases the surface area.
(ii) The Nature of the Adsorbed Gas
The extent of adsorption also depends upon the
nature of the gas. The gases which are more easily liquifiable or are more
soluble in water are more readily adsorbed than others. For example, under
similar conditions, the amount of SO2 or NH3 adsorbed by
charcoal is much more than that of H2 or O2 gases. It is
because the intermolecular forces are stronger in more easily liquifiable
gases, therefore, they get adsorbed more strongly.
(iii) Temperature
The extent of adsorption decreases with rise in
temperature. For example, under one atmosphere pressure, one gram of charcoal
adsorbs about 10 cm3 of N2 gas at 272 K, 20 cm3
at 248 K and 45 cm3 at 195 K.
Adsorption
is an exothermic process. The change in enthalpy when one mole of a substance
is adsorbed, is called enthalpy of
adsorption. The adsorption process is similar to the condensation process. The reverse process is called
desorption and is endothermic in nature. It is similar to
the evaporation process. When a gas is kept in contact with a solid adsorbent in a closed container, a dynamic
equilibrium is established in due course of time.
gas adsorbate + solid adsorbent ↔ gas adsorbed on the solid + heat
Since the forward process (adsorption) is
exothermic in nature, according to the Le Chatelier's principle, it would be
favoured at low temperature. Therefore, the extent of adsorption would increase
on decreasing the temperature and would decrease on increasing the temperature.
(iv) Pressure of the gas
At a constant temperature the extent of adsorption
increases with increase in the pressure of the gas (adsorbate). We shall study
the relation between the two in detail a little later.
Related Topics