Chapter: Engineering Chemistry: Surface Chemistry and Catalysis
Adsorption Isotherms
Adsorption
Isotherms
The extent of adsorption is measured in terms of
the quantity x/m where, x is the mass of the gas (adsorbate)
adsorbed at equilibrium on mass m of the adsorbent. x/m is the mass of the adsorbate adsorbed per
unit mass of the adsorbent. The graph showing variation in x/m with pressure(p)
at a constant temperature is called adsorption isotherm. Let us see the
variation in extent of adsorption in case of gases and of solutes from their solutions.
(i) Adsorption of Gases
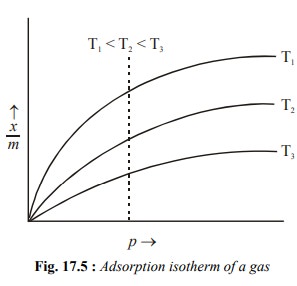
The adsorption isotherm of a gas which is adsorbed
on a solid is shown in Fig. 17.5. It shows that the extent of adsorption of a
gas on a solid increases with the increase in the pressure of the gas, p at three different constant
temperatures. The curves also show that the extent of adsorption, decreases at
a fixed pressure as the temperature is increased (see the dotted line).
Freundlich Adsorption Isotherm
Freundlich gave an empirical mathematical
relationship between the extent of adsorption (x/m) and the equilibrium
pressure (p) of the gas as :
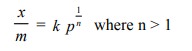
In this relation k is a constant at a given temperature and depends upon the nature
of the adsorbate and adsorbent. The value of n changes with pressure. It is 1 at low pressures and increases
with pressure. The relationship is valid at a constant temperature. Therefore,
it is called Freundlich Adsorption
Isotherm. On taking logarithm of the above equation, we get
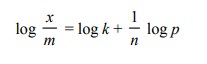
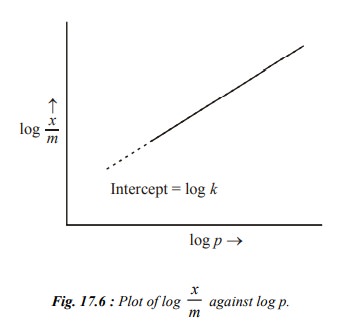
This is an equation of a straight line and a plot
of log x/m against log p should be a straight line with slope 1/n as depicted
in Fig. 17.6. In actual practice, a straight line is obtained provided the data
at very low and very high pressures is neglected.
Related Topics