Engineering Chemistry - Adsorption | Engineering Chemistry: Surface Chemistry and Catalysis
Chapter: Engineering Chemistry: Surface Chemistry and Catalysis
Adsorption
Adsorption
The surface of a solid attracts and retains
molecules of a gas or a dissolved substance which comes in its contact. These
molecules remain only at the surface and do not go deeper into the bulk Fig.
17.2(a).
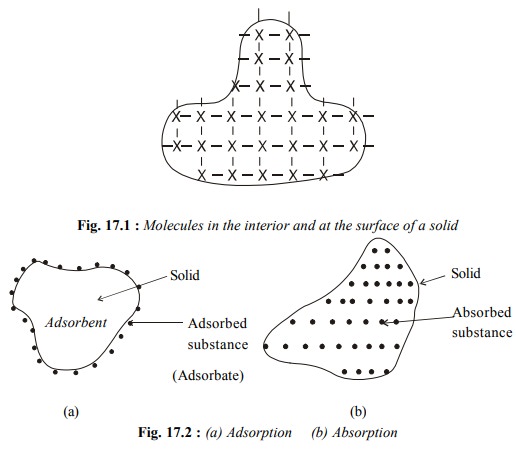
The
phenomenon of attracting and retaining the molecules of a gas or a dissolved
substance by the surface of a solid, resulting in their higher concentration on
the surface is called adsorption.
The substance which gets adsorbed is called the adsorbate and the solid substance which
adsorbs is called the adsorbent.
A molecule in the interior of a solid is surrounded by other molecules in all directions (Fig. 17.1). However, a molecule at the surface is surrounded by other molecules within the solid phase but not from the outside.Therefore, these surface molecules have some unbalanced or residual forces.
Related Topics