Chapter: Medical Immunology: Diagnostic Immunology
Laboratory Tests For Assessment of Lymphocyte Function
LABORATORY TESTS FOR ASSESSMENT OF LYMPHOCYTE FUNCTION
The evaluation of cell-mediated immunity presents considerable more difficulties than the evaluation of the humoral immune responses. In vivo tests, such as skin tests with common antigens known to induce delayed hypersensitive reactions, are difficult to standardize. In vitro functional tests are difficult to execute, time-consuming, and require specialized per-sonnel and sophisticated equipment.
A. Isolation of Mononuclear Cells
All lymphocyte functional assays require at least partial isolation of a lymphocyte-en-riched mononuclear cell population (mononuclear cells include lymphocytes, NK cells, and monocytes). This is usually achieved by density gradient centrifugation, usually in Ficoll-Hypaque. This separation medium has a specific gravity of 1.077, which lies be-tween the density of human erythrocytes (1.092) and the density of human lymphocytes (1.070). By carefully centrifuging blood in Ficoll-Hypaque, a gradient is formed with erythrocytes and polymorphonuclear leukocytes sedimented at the bottom of the tube, a thin layer containing lymphocytes and monocytes appears in the interface between Ficoll-Hypaque and plasma, and platelet-rich plasma fills the rest of the tube from the mononu-clear cell layer to the top. Approximately 80% of the cells recovered in the mononuclear cell layer are lymphocytes and 20% are monocytes. Of the lymphocytes, approximately 80% are T cells, 4–10% are B cells, and the remaining are NK cells and other non-T, non-B lymphocytes.
B. Mitogenic Stimulation Assays
Human lymphocytes can be stimulated in vitro by specific antigens or by mitogenic sub-stances. Although testing the response to specific antigens should be the preferred approach to the study of lymphocyte function, the likelihood of success in such studies is limited by the fact that very few T cells (and even fewer B cells) in the peripheral blood will carry spe-cific receptors for any antigen, even if the individual has already developed a memory re-sponse to that particular antigen. In contrast, mitogenic responses are easier to elicit be-cause the mitogenic substances are able to stimulate nonspecifically large numbers of peripheral blood lymphocytes, and therefore lymphocyte proliferation becomes much eas-ier to detect. The most widely used mitogens are plant glycoproteins (lectins), such as phy-tohemagglutinin (PHA), concanavalin A (ConA), and pokeweed mitogen (PWM). PWM stimulates both B cells and T cells, while PHA and ConA stimulate T cells only. Immobi-lized anti-CD3 monoclonal antibodies also have mitogenic properties. When immobilized on a tissue culture plate, for example, these antibodies cross-link multiple TcR complexes on the T-lymphocyte membrane and deliver a mitogenic signal to CD3+ T lymphocytes. The immobilization of the monoclonal antibody can be easily achieved by spontaneous ad-sorption to the walls of the microculture plates used in proliferation assays or by binding to monocytes present in the mononuclear cell culture through Fc receptors. The use of anti-CD3 as a mitogen has the advantage of probing the function of the transducing component of the T-cell receptor
These mitogenic stimulation assays are performed with mononuclear cell suspen-sions, usually adjusted at 1x106 lymphocytes/mL, to which a stimulating compound is then added, usually in two or three different concentrations. Upon stimulation, T lympho-cytes are activated and eventually undergo differentiation. Several endpoints are used to de-tect lymphocyte activation:
a. Incorporation of tritiated thymidine into dividing cells is the most common end-point used to measure lymphocyte proliferation. In the stage of blastogenic trans- formation there is intense lymphocyte proliferation with active DNA synthesis. Tritiated thymidine [3H-Tdr] is added to the culture after 72 hours of incubation with the mitogen, and the dividing cells remain exposed to 3H-Tdr for 6–8 hours. The lymphocytes are then harvested, washed, and the amount of radioactivity in- corporated into DNA by the dividing cells is determined with a scintillation counter. A stimulation index (SI) can be calculated as follows:
SI = cpm in mitogen-stimulated lymphocytes / cpm in unstimulated control lymphocytes
b. Immunoglobulin synthesis can be easily measured by determining the concen-trations of IgG and IgM in supernatants harvested 5–6 days after PWM stimula-tion using RIA or EIA. This is the best endpoint when B-cell function is to be evaluated.
c. The identification of cytokines was quickly followed by the development of monoclonal antibodies, which became the basis for of enzymoimmunoassays that have rapidly replaced most functional assays. The availability of these as-says has provided a physiological endpoint for studies of T-lymphocyte acti-vation. (a) The assay of IL-2 by EIA is probably the method of choice for the evaluation of the initial stages of activation of the T helper cell population. The most common approach to this assay consists of incubating mononuclear cells with several concentrations of mitogenic substances for 24 hours and measuring IL-2 concentrations in the supernatants. Low or absent release of IL-2 has been observed in a variety of immunodeficiency states, particularly in patients with AIDS. (b) Expression of IL-2 receptors can be detected 24 hours after mitogenic stimulation. The expression of IL-2 receptors can be measured by flow cytometry, using fluorescent-labeled anti-CD25 (TAC) anti-bodies. Both T cells and B cells overexpress CD25 after activation, but with the proper combination of monoclonals, flow cytometry allows the separate de-termination of activated B cells and activated T cells. (c) Enzymoimmunoas-says for IL-4, IL-5, IL-6, IL-10, IL-12, GM-CSF, TNF, LTα , and interferon-are also available, and their judicious use allows us to obtain a more complete picture of the functional response of T lymphocytes. For example, predominant release of IL-4, IL-5, and IL-10 is characteristic of TH 2 responses, while pre-dominant release of IL-2, GM-CSF, and interferon-γ is characteristic of TH1 responses.
d. Cytokine mRNA assays are gaining popularity particularly in the analysis of TH1 vs. TH 2 responses. Several techniques are available, all requiring suitable cDNA probes, which can be isotopically or nonisotopically labeled. Some techniques are based on hybridization of PCR-amplified mRNA obtained from nonstimulated T-lymphocyte clones; others are based on in situ hybridization performed on slides of stimulated cells. The current techniques are semi-quan-titative at best, but progress in this area has been very rapid. Cytokine produc-tion by defined cell populations can also be evaluated by flow cytometry (see Sec. I.E.). This method has several advantages over mRNA analysis; it is rapid, quantitative, and allows for the simultaneous measurement of several cytokines and surface markers.
Mitogenic responses are relatively simple to study, but the assays have a variety of problems, such as poor reproducibility and individual variations among normal individuals. In addition, these assays only measure the prolifera-tive capacity of lymphocytes and are not very informative about their functional activity. Nevertheless, the finding of a very low mitogenic index after stimula-tion with a T-lymphocyte mitogen suggests a deficiency of T-cell function.
C. Response to Antigenic Stimulation
The study of the response of lymphocytes to antigenic stimulation in vitro is functionally more relevant than the study of mitogenic responses. However, even in the best possible circumstances, i.e., when the antigen can be recognized by T lymphocytes, which predom-inate in peripheral blood, and the lymphocyte donor has developed memory to the antigen in question, the proportion of cells responding to stimulation is likely not to exceed 0.1%, and the proportion of responding B lymphocytes is even lower. The probabilities of ob-taining a measurable response may be increased when the lymphocytes are stimulated with antigens to which the lymphocyte donor has been previously exposed and the cultures are incubated with the antigen for 5–7 days prior to addition of 3H-Tdr. However, this elimi-nates the possibility of determining whether the patient can mount a primary response.
The elicitation of B lymphocyte responses in vitro is considerably more difficult. Most studies in which positive results have been reported have used heterologous red cells or tetanus toxoids as antigens. The in vitro response to tetanus toxoid is easier to elicit us-ing peripheral blood mononuclear cells separated from donors who had received a booster 1–3 weeks earlier. Usually, the incubation periods in studies of B-cell activation have to be increased even further, up to 9–11 days.
The antigens most commonly used in these studies are purified protein derivative (PPD), Candida albicans antigens, keyhole limpet hemocyanin, and tetanus toxoid (which stimulates both T and B cells), none of which has been properly standardized. In addition, some of these antigens seem to have mitogenic properties, and when 3HTdr incorporation is the endpoint measured, it is not possible to determine whether the response is due to anti-genic or mitogenic stimulation.
D. Tetramer Assays
One of the developments with greater potential significance in the last few years has been the development of MHC/peptide tetramers. Both MHC-I and MHC-II tetramers able to bind specific peptides have been constructed, and once loaded with a peptide, they interact specifically with CD8+ or CD4+ T cells with TcR able to recognize the peptide-MHC com-bination in question. For example, MHC-I–peptide complexes loaded with peptides de-rived from melanoma-associated antigens have been shown to be able to bind specifically to CD8+ lymphocytes from melanoma patients, which can be then purified and shown to be able to lyse melanoma tumor cells in vitro. On the other hand, MHC-II tetramers loaded with an influenza hemagglutinin (HA) peptide have been shown to bind and activate HA-specific CD4+ T cells. The potential for application of this technique to characterize nor-mal and abnormal aspects of cell-mediated immune responses appears almost unlimited.
E. Assay of Cytokines and Cytokine Receptors in Plasma and Urine
While antigenic and mitogenic stimulation assays test lymphocyte reactions under more or less physiological conditions, the target cells are those present in circulation, and it can be argued that the sampling does not adequately reflect the state of activation of their tissue counterparts. This is particularly problematic when the objective is to assess role of the T-cell system in a patient with a hypersensitivity disease. Several groups have proposed that the assay of circulating cytokines or cytokine receptors (shed as a consequence of cell ac-tivation) is more reflective of the state of activation of T cells in vivo. It must be noted that these assays have not proven to be as useful as expected. A major limiting factor is their lack of disease specificity, which makes their correlation with specific clinical conditions rather difficult. In addition, the high cost of reagents is a significant limiting factor that has prevented their widespread use and proper evaluation of their usefulness.
1. Serum IL-2 levels have been measured as a way to evaluate the state of T-lym-phocyte activation in vivo. Increased levels of circulating IL-2 have been re-ported in multiple sclerosis, rheumatoid arthritis, and patients undergoing graft rejection, situations in which T-cell hyperactivity would fit with the clinical pic-ture. However, a significant problem with these assays is the existence of a serum factor that interferes with the assay of IL-2 by EIA, and the results are of-ten very imprecise.
2. Urinary levels of IL-2 can also be measured by enzymoimmunoassay and are not affected by inhibitory substances. Increased urinary levels of IL-2 have been re-ported in association with kidney allograft rejection and proposed as a parame-ter, which may help differentiate acute rejection from cyclosporin A toxicity. However, this approach has not gained widespread accep-tance.
3. Activated T cells shed many of their membrane receptors, including the IL-2 re-ceptor. Elevated levels of circulating soluble receptors (shed by activated T lym-phocytes) have been found in patients with hairy cell leukemia, AIDS, rheuma-toid arthritis, graft rejection, etc. In general, the results of assays for IL-2 receptor parallel the results of assays for IL-2.
4. The measurement of serum IL-6 correlates with B lymphocyte activity and in-flammation. High levels of IL-6 have been detected in patients with AIDS, sys-temic lupus erythematosus, and systemic inflammatory reactions.
F. Assays for Cytotoxic Effector Cells
The functional evaluation of cytotoxic effector cells, which include cytotoxic T lympho-cytes, natural killer (NK) cells, and cells mediating antibody-dependent cell cytotoxicity (ADCC) reactions, is based on cytotoxicity assays. The functional interest of cytotoxicity assays is evident. In the case of T cells, cytotoxicity assays measure the functional ade-quacy of one of the major effector T-cell subpopulations. As for NK and ADCC effector cells, their functional definition requires functional assays.
1. The cellular targets for cytotoxic cells vary according to cytotoxic cell population to be evaluated:
2. The evaluation of T-cell–mediated cytotoxicity requires mixing sensitized cytotoxic T cells with targets expressing the sensitizing antigen.
3. NK-cell activity is usually measured with tumor cell lines known to be susceptible to NK-cell killing.
4. ADCC is measured using antibody-coated target cells.
Two main methods are used to assay cytotoxicity:
1. Counting of dead cells—dead target cells are differentiated from live target cells by the uptake of vital dyes, such as trypan blue. This technique, however, is mostly used for the study of antibody-mediated cytotoxicity.
2. Release of radiolabeled chromium (51Cr) from previously labeled target cells is pre-ferred when evaluating T-lymphocyte or NK-cell cytotoxity.
G. Mixed Lymphocyte Reaction
One of the most informative tests for the evaluation of T-lymphocyte function in vitro is the mixed lymphocyte reaction (MLR). The basis of the MLR is the recognition of anti-genic differences mostly related to the expression of class II MHC antigens on the mem-brane of mononuclear cells. The endpoint of the test may be 3HTdr incorporation or 51Cr release from target cells. It must be stressed that MLR tests are difficult and time consum-ing and have very limited, if any, clinical use.
One-way MLR reactions are tests in which one of the cell populations is treated with a DNA synthesis blocker so that it acts as a stimulator of non-HLA identical cells, while the other population is untreated and acts as the responder population. These reactions were used for many years to type HLA-D locus specificities, the first class II HLA specificities described. This was usually accomplished by mixing mitomycin-treated mononuclear cells from a donor of known HLA-D specificity with untreated mononuclear cells of an untyped individual (Fig. 15.17). A response in this system, measured by 3HTdr incorporation, was considered as indicating lack of identity of the D locus. This cumbersome approach has been made obsolete by the development of antibodies and DNA probes to type MHC-II specificities .
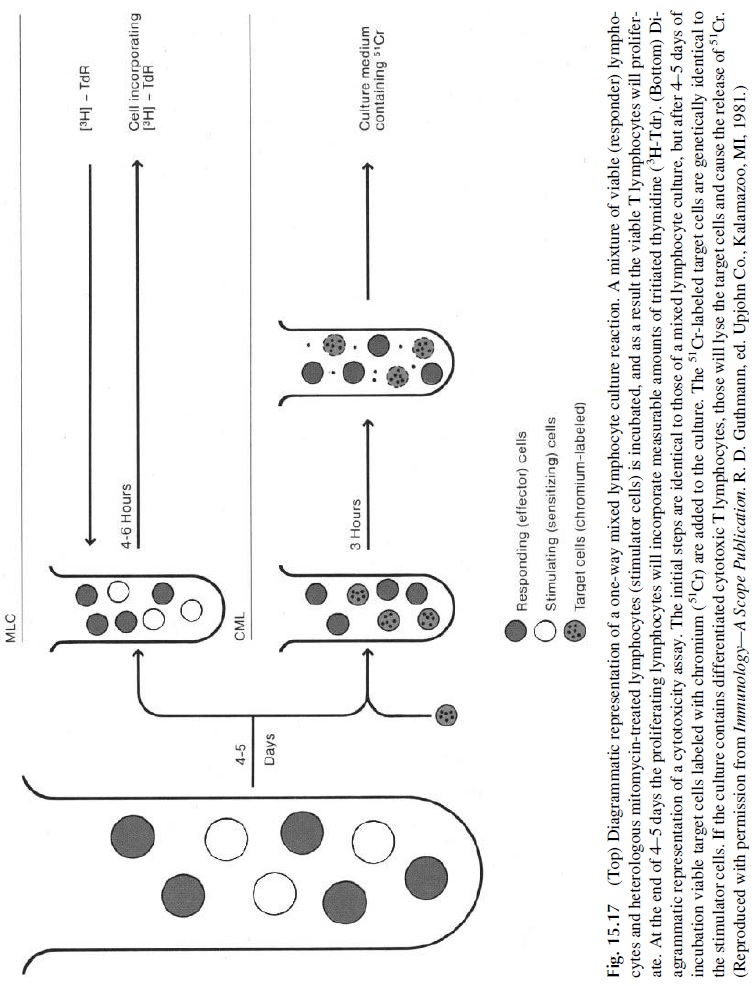
The one-way MLR can also be used to evaluate cytotoxic T-cell function. In this case mitomycin-treated mononuclear cells from a genetically unrelated donor are used both as stimulators and as targets of a patient’s lymphocytes. The MLR is set as a mixed culture of inactivated stimulator cells and responder T cell. The presence of MHC-II cells in the mixture is essential, because the responding CD4+ cells can only be activated by nonself peptides presented by nonpolymorphic MHC-II molecules shared by most individuals of the species. After incubating the culture for five days, 51Cr-labeled viable “target” lym-phocytes, obtained from the same individual who provided the cells used as stimulators, are added to the culture. If cytotoxic T cells were generated during the previous 5 days of cul-ture, the viable cells added in the second step will became their targets and will be killed in a few hours, releasing significant amounts of 51Cr (Fig. 15.17).
Two-way MLRs are set by mixing untreated mononuclear cells from genetically unre-lated individuals. This approach is usually used to determine the compatibility of two unre-lated individuals, such as when donors for a bone marrow graft are to be screened. 3HTdr is added after 4–5 days to the culture to determine whether the cells are proliferating. The lack of significant 3HTdr incorporation indicates that the donor and recipient are compatible.
Related Topics