Chapter: Medical Immunology: Diagnostic Immunology
Assay Methods Based on Agglutination - Immunoserology
Assay Methods Based on Agglutination
1. Bacterial Agglutination
When a bacterial suspension is mixed with antibody directed to its surface determinants, the antigen-antibody reaction leads to the clumping (agglutination) of bacterial cells.
Agglutination is rapid (taking a matter of minutes) and, by being visible to the naked eye, does not require any special instrumentation other than a light box. Its disadvantages are the need for isolated organisms and poor sensitivity, requiring relatively large concen-trations of antibody.
In spite of its limitations, agglutination of whole microorganisms is commonly used for serotyping isolated organisms and, less commonly, for the diagnosis of some infectious diseases, e.g., the Weil-Felix test for the diagnosis of typhus (based on the fact that certain strains of Proteus share antigens with several Rickettsia species). Methods based on the ag-glutination of antigen or antibody-coated particles are more widely used in diagnostic im-munology.
2. Agglutination of Inert Particles Coated with Antigen or Antibody
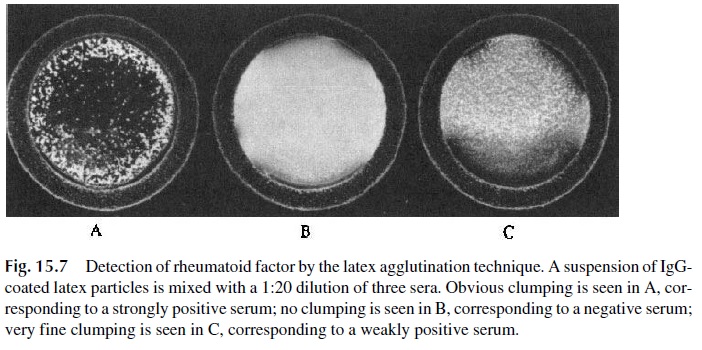
Latex particles and other inert particles can be coated with purified antigen and will agglu-tinate in the presence of specific antibody. Conversely, specific antibodies can be easily ad-sorbed by latex particles and will agglutinate in the presence of the corresponding antigen. Quantitative analysis to determine the agglutinating antibody content of an antiserum in-volves dilution of the serum and determination of an endpoint, which is the last dilution at which agglutination can be observed. The reciprocal of this last agglutinating dilution is the antibody titer.
This methodology has found a variety of applications:
· IgG-coated, latex particles are used for the detection of anti-immunoglobulin factors (such as the rheumatoid factor) in the rheumatoid arthritis (RA) test (Fig. 15.7).
· Latex particles coated with thyroglobulin are used in a thyroglobulin antibody test.
· Several diagnostic tests for infectious diseases have been developed based on latex agglutination. In some cases, the antigen is bound to latex, and the test detects specific antibodies (e.g., tests for histoplasmosis, cryptococcosis, and trichi-nosis) Rapid diagnosis tests for bacterial and fungal meningitis have been de-veloped by adsorbing the relevant specific antibodies to latex particles. The an-tibody-coated particles will agglutinate if mixed with CSF containing the relevant antigen. This procedure allows a rapid etiological diagnosis of menin-gitis that is essential for proper therapy to be initiated.
The main advantages of these tests are simplicity and the quick turnaround of results. The main disadvantages are the need for large amounts of reagents, cost, and relatively low sensitivity, particularly in the case of the tests for diagnosis of infectious diseases.
3. Hemagglutination
Hemagglutination, i.e., red cell agglutination, is the basis of a wide array of serological tests that can be subclassified depending on whether they detect antibodies against red cell de-terminants (direct and indirect hemagglutination) or against compounds artificially coupled to red cells (passive hemagglutination).
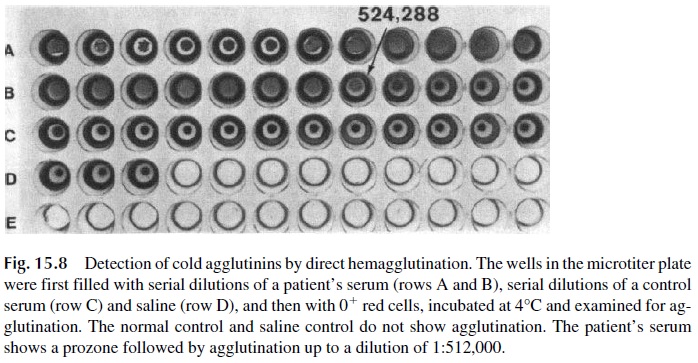
Direct hemagglutination tests are carried out with washed red cells that are aggluti-nated when mixed with IgM antibodies recognizing membrane epitopes. For example, di-rect agglutination tests have been used for the determination of the ABO blood group and titration of isohemagglutinins (anti-A and anti-B antibodies); for the titration of cold hemagglutinins (IgM antibodies, which agglutinate red blood cells at temperatures below that of the body), as illustrated in Figure 15.8; and for the Paul-Bunnell test, useful for the diagnosis of infectious mononucleosis. This last test detects circulating heterophile anti-bodies (cross-reactive antibodies that combine with antigens of an animal of a different species) that induce the agglutination of sheep or horse erythrocytes.
Hemagglutination is simple to execute and requires very simple materials. However, the tests for cold agglutinins associated with infectious diseases and the Paul-Bunnell test lack specificity.
Indirect hemagglutination is used to detect antibodies that react with antigens present in the erythrocytes but which by themselves cannot induce agglutination. Usually these are IgG antibodies that are not as efficient agglutinators of red cells as polymeric IgM anti-bodies. A second antibody directed to human Immunoglobulins is used to induce aggluti-nation by reacting with the red cell–bound IgG molecules and, consequently, cross-linking the red cells.
The technique is simple to perform. Poor sensitivity is the main limitation. The best known example of indirect agglutination is the antiglobulin or Coomb’s test, which is used in the diagnosis of autoimmune hemolytic anemia .
Passive hemagglutination techniques use red blood cells as a substrate, much as la-tex is used in tests involving inert particles. Antigen can be coated onto the red cells by a variety of methods, and the coated cells will agglutinate when exposed to specific antibody. This system was used as the basis for a variety of diagnostic procedures, such as a test to detect antithyroid antibodies, the Rose-Waaler test for anti-Ig factors present in the serum of patients with rheumatoid arthritis, and many tests to detect anti-infectious antibodies. However, it has been progressively replaced by more sensitive and less time-consuming techniques.
Hemagglutination inhibition was used in a variety of ways for antigen and antibody assays, but the only clinical application that remains is the semi-quantitative assay of anti-bodies to influenza viruses. The influenza virus has envelope glycoproteins that agglutinate avian red blood cells (viral hemagglutinins). The immune response against the virus in-cluded antibodies that combine with these hemagglutinins and prevent red cell agglutina-tion. A semi-quantitative assay of viral antibodies can be performed by determining the maximal dilution of serum that can inhibit viral hemagglutination. A fourfold or greater in-crease in titer observed in two samples collected from the same patient in an interval of 2–3 weeks is considered indicative of a recent infection. The assay is not very useful clinically but is essential to confirm the diagnosis of viral influenza and for the epidemiological surveillance of this infection.
Related Topics