Chapter: Civil : Construction Materials: Lime, Cement, Aggregates, Mortar
Heat of hydration
Heat of hydration
Heat is evolved during hydration
of cement, the amount being dependent on the relative quantities of the clinker
compounds.
Importance: The evolution of heat
causes an increase in temperature of the concrete, being greatest in mass concreting.
Since the cooling of a mass of concrete can only occur from surfaces exposed to
atmosphere the temperature of the interior is higher than that at the surface
and also there is a rapid increase in strength in the interior than at the
surface. Shrinkage cracks may result from stresses, induced by cooling of the
surface while the interior of concrete is still at higher temperature. However,
in practice, the heat evolution may be taken to its advantage in cold weather
provided the concrete is warm at the time of placing and excessive heat loss is
prevented by suitable lagging.
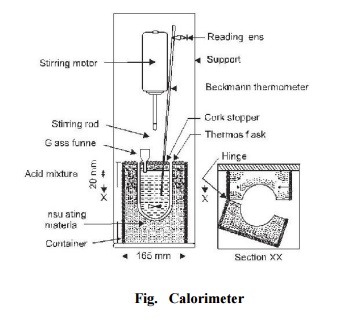
Test Procedure: The apparatus
used to determine the heat of hydration of cement is known as calorimeter and
is shown in Fig. 32. 60 g of cement and 24 ml of distilled water are mixed for
4 minutes at temperature 15 o -25 o C. Three specimen glass
vials 100 × 20 mm are filled with this mixture, corked and sealed with wax. The
vials are then stored with the mixture in a vertical position at 27 o ±2 o C. The
heat of hydration is obtained by subtracting the respective heat of solution of
unhyrated cement calculated nearest to 0.1 calorie.
For
determining the heat of solution of unhydrated cement, weigh a sample of about
3 g. At the same time, weigh out 7.0 g of cement for the loss on ignition.
Heat of solution (Cal/g) of unhydrated cement
where 0.2 is the specific heat of unhydrated cement.
For determining heat of solution
of the hydrated cement, one of the glass vials is opened and the adherent wax
is removed. The cement is ground rapidly, to avoid carbonation, to pass an 850
micron sieve. From this weigh out 4.2 g and 7.0 g of cement samples for heat of
solution and loss on ignition.
The heat
of solution of hydrated cement (Cal/g ignited weight)
Heat capacity
× corrected temperature
/ Weight of sample corrected for ignition M 0 M)
The ignition loss can be obtained
by placing the sample in a cool furnace and raising the temperature of the
furnace to 900 o C over a period of 1 hour. The sample is kept at 900 o ± 50 o C for
3 -4 hours and then cooled in a desiccator containing anhydrous calcium
chloride. Weigh after half an hour. The difference in the two weighings give
the loss on ignition.
To
determine the heat capacity sufficient quantity of zinc oxide is ignited for
one hour at 900 o ± 50 o C. It is cooled in a desiccator containing anhydrous
calcium chloride and ground to pass 250 micron sieve. About 7 g of this ignited
oxide is reheated to 900 o ± 50 o C for 5 minutes and then cooled for about 2½
hours (not more than 5 hours). The calorimeter is assembled and temperature
reading correct to 0.001 o C is recorded to determine the initial heating or
cooling correction. The zinc oxide is then introduced. The temperature readings
are recorded at one minute intervals until the solution is complete. The
recording of readings is continued for next 5 minutes to determine the final
heating or cooling correction. The initial and final heating or cooling rates
against the corresponding calorimeter temperature are plotted. The two points
thus obtained are joined by a straight line. From this graph the corrections
are read off for each temperature reading during the solution period. Heat
capacity is calculated from the expression.
Heat capcity (Cal/ o C)
= Weight of ZnO / Corrected temperature rise [256.10.1(30.0 \ 0 ) 0.1(\ 0
= Weight of ZnO (259.1 0.2\ 0.1\0 ) / Corrected
temperature rise
where, 256.1 is the heat of solution
of zinc oxide at 30 o C and 0.2 the negative temperature coefficient of the heat
of solution, is the final temperature of the calorimeter, 0.1 is the specific
heat of zinc oxide and is the room temperature in o C.
Related Topics