Chapter: Civil : Construction Materials: Lime, Cement, Aggregates, Mortar
Composition Of Cement Clinker
Composition Of Cement Clinker
The
various constituents combine in burning and form cement clinker. The compounds
formed in the burning process have the properties of setting and hardening in
the presence of water. They are known as Bogue compounds after the name of
Bogue who identified them. Le-Chatelier and Tornebohm have referred these
compounds as Alite (C3S), Belite (C2S), Celite (C3A)
and Felite (C4AF). The following Bogue compounds are formed during
clinkering process.
The properties of Portland cement
varies markedly with the proportions of the above four compounds, reflecting
substantial difference between their individual behaviour.
Tricalcium Silicate is
supposed to be the best cementing material and is well burnt cement. It is
about 25-50% (normally about 40 per cent) of cement. It renders the
clinker easier to grind, increases resistance to freezing and thawing, hydrates
rapidly generating high heat and develops an early hardness and strength.
However, raising of C3S content beyond the specified limits
increases the heat of hydration and solubility of cement in water. The
hydrolysis of C3S is mainly responsible for 7 day strength and
hardness. The rate of hydrolysis of C3S and the character of gel
developed are the main causes of the hardness and early strength of cement
paste. The heat of hydration is 500 J/g.
Dicalcium Silicate is about
25-40% (normally about 32 per cent) of cement. It hydrates and hardens slowly
and takes long time to add to the strength (after a year or more). It imparts
resistance to chemical attack. Raising of C2S content renders
clinker harder to grind, reduces early strength, decreases resistance to
freezing and thawing at early ages and decreases heat of hydration. The
hydrolysis of C2S proceeds slowly. At early ages, less than a month,
C2S has little influence on strength and hardness. While after one
year, its contribution to the strength and hardness is proportionately almost
equal to C3S. The heat of hydration is 260 J/g.
Tricalcium Aluminate is about
5-11% (normally about 10.5 per cent) of cement. It rapidly reacts with water
and is responsible for flash set of finely grounded clinker. The rapidity of action
is regulated by the addition of 2-3% of gypsum at the time of grinding cement.
Tricalcium aluminate is responsible for the initial set, high heat of hydration
and has greater tendency to volume changes causing cracking. Raising the C3A
content reduces the setting time, weakens resistance to sulphate attack and
lowers the ultimate strength, heat of hydration and contraction during air
hardening. The heat of hydration of 865 J/g.
Tetracalcium Alumino Ferrite is about
8-14%
(normally about 9 per cent) of cement. It is responsible for flash set
but generates less heat. It has poorest cementing value. Raising the C4AF
content reduces the strength slightly. The heat of hydration is 420 J/g.
Calculation of Compound Composition
of Portland Cement: Bogue developed a method for calculating
the compound composition from the oxide analysis of a cement. This method is
based upon cooling of the clinker at such rate that equilibrium is maintained.
Although equilibrium does not usually obtain in commercial operations, valuable
information can be derived from such calculations. The method is summarized in
the following steps and in Table 5. An accurate chemical analysis is entered in
the first column of the table as shown.
Table 5
Record of Significant Data for Computing Compound Composition
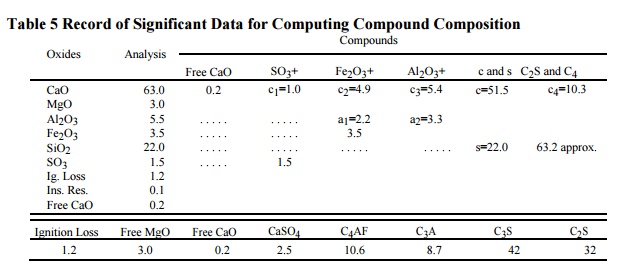
Since the
ratio of the atomic weight of CaO : SO3 = 56.07 : 80.065 = 0.70:1,
each percentage of SO3 combines with 0.70 per cent of Cao to form
1.70 per cent of CaSO4. Hence, the percentage of lime required to
satisfy SO3 (= 0.7 × per cent SO 3) is recorded as c1
in column 4 of the table, opposite CaO; the percentage of SO3 is
also entered in column 4; and the CaSO4 content is summed and
entered at the bottom of column 4.
Similarly, since the atomic
ratios Al2O3 : Fe2O3 = 101.92 :
159.68 = 0.64 : 1 and 4CaO : Fe2O3 = 224.28 : 159.68 =
1.40 : 1, it is evident that each percentage of Fe2O3
enters into combination with 0.64 per cent of Al2O3 and
1.40 per cent of CaO to form 3.04 per cent of 4CaO × Al 2O3
× Fe 2O3. Therefore 0.64 × percentage of Fe 2O3
is entered as a1, opposite Al2O3 in column 5,
and 1.4 × percentage of Fe 2O3 is entered as c2,
opposite CaO in column 5; the percentage of Fe2O3 is
re-entered in the same column; and the percentage of C4AF is summed
at the bottom.
Since practically none of the magnesia is combined, it is
entered as free at the bottom of column 2. The total alumina minus a1,
entered as a2 in column 6, is available to combine with lime to form
C3A
in the ratio 3CaO : Al2O3
= 168.21 : 101.92 is 1.65 : 1. Hence, each percentage of this available alumina
× 1.65 is the percentage of CaO required for C 3A, and it is entered
opposite CaO as c3 in column 6. Summing quantities in column 6 gives
percentage of C3A.
The CaO available to combine with
SiO2 is total CaO minus (free CaO + c1 + c2 +
c3); call this difference c. Then the total silica (s) is calculated
first to combine with CaO to form C2S. Since the ratio 2CaO × SiO 2
: SiO2 = 172.20 : 60.06 = 2.87:1, each percentage of s × 2.87 is the
percentage of C 2S. This first approximation of C2S is
entered in column 8, opposite SiO2. By subtracting this value of C2S
from the sum s + c, the amount of CaO (called c4) available for
combination with 2CaO × SiO 2 to form 3CaO × SiO 2 is
determined. Since the ratio 3CaO × SiO 2:CaO = 228.27:56.07 =
4.07:1, multiplying c4 by 4.07 gives the amount of C3S
which is entered at the foot of column 7. By subtracting this value of C3S
from c + s, the true percentage of C2S is found and entered in
column 8.
Should the computed percentage of
C3S be greater than c + s, no C2S is present. In that
case the content of C3S is found from the ratio 3CaO × SiO 2
: SiO2 = 228.27 : 60.06 = 3.8 : 1. Hence, the percentage of C3S
is obtained by multiplying the percentage of SiO2 by 3.8. This
latter value of C3S, subtracted from c + s, gives the percentage of
uncombined lime. This last condition can only be obtained when lime is in
excess of the amount required for equilibrium and the free lime has not been
deducted.
Since errors in chemical analysis
of 0.2 per cent in determinations of lime, alumina, silica, or iron oxide will
make errors up to 1.5 per cent in certain compounds, percentages for the
compounds should be rounded off to whole numbers. If the ignition loss is high,
the analysis should be reduced to a clinker basis prior to compound
calculations.
As previously mentioned, Bogue's
method of calculation is based on the assumption that the clinker is slowly
cooled at such rate that equilibrium is maintained and the crystallization is
complete. Lea and Parker have shown that values calculated by the bogue method
may be considerably in error if the clinker liquid crystallizes independently
of the solids formed, or if cooling is so sudden that no crystallization takes
place and glass is formed. For the case of independent crystallization and a
clinkering temperature of 1400 o C, they show that Bogue's method is correct for
cements with Al2O3/Fe2O3 ratios
between 0.9 and 1.7, but for ratios between 1.7 and 6.1 their corrections to be
added are :
C3S, + (1.8Al2O3 - 2.8Fe2O3)
C2S, + (2.1Fe2O3 - 1.4Al2O3)
C3A, + (2.5Fe2O3
- 1.6Al2O3)
C4AF, Nil
Thus for a cement with Al2O3
= 7 and Fe2O3 = 3 per cent, the correction to C3S
= 4.2, to C2S = -3.5, and to C3A
= -3.7 per
cent.
For very rapid cooling of the
clinker, the liquid is formed into glass and they show that no C3A
or C4AF appear but the amount of glass is + (2.95Al2O3
+ 2.2Fe2O3). For this case their corrections to Bogue's
values for C3S and C2S are: C3S, + (1.8Al2O3
- 2.8Fe2O3);
C2S + (1.9Fe2O3
-
2.1Al2O3).
Related Topics