Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Early life history
Getting from here to there: larval transport mechanisms
Getting from here to there: larval transport mechanisms
Many inshore marine fishes in temperate and tropical environments spawn offshore but their larvae or juveniles use shallow habitats such as bays, mangroves, and other estuarine regions as nurseries. This characterizes many coral reef species and anguillid eels, croakers, porgies, Bluefish, scorpionfishes, and flatfishes among temperate species. An important question therefore is how do such larvae, with their relatively limited swimming capabilities, move to shallow habitats? For coral reef species, many of which spawn at reef edges, luck may combine with behavior to determine dispersal. Active orientation, directed movement, utilization of favorable currents, and habitat choice are all implied by distribution and behavior of some species (Leis 1991; Kaufman et al. 1992; Cowen & Castro 1994; see above, Larval behavior and physiology).
For species along continents, however, adults may spawn 100 km or farther offshore, and larvae must traverse the continental shelf to arrive at nursery grounds in 2 or 3 months. Given that the average fish larva can swim only about 1 to 2 body lengths per second, or about 1 km/day, active processes such as directed swimming will be too expensive energetically as well as too slow to transport larvae such extensive distances or to fi ght the strong, outflowing currents that characterize embayments, sounds, passes, and many other estuarine locales. Additionally, once larvae find their way to a nursery ground, they frequently remain in specific regions, again requiring that they fight strong tidal, river, and wind currents that should flush them back out to sea. Behavioral adaptations of the larvae themselves are therefore implicated in finding and remaining in appropriate habitats.
The larval transport phenomenon has three main components: movement towards shore, location of and movement into nursery areas, and retention in nursery areas (Norcross & Shaw 1984; Boehlert & Mundy 1988; Miller 1988) (Fig. 9.11). Most interpretations of distribution patterns and behavior propose a combination of passive and active mechanisms. The degree of passivity decreases with age. Although young larvae may rely largely on passive transport of the water mass in which they hatched, older larvae can actively seek particular water masses with which they move. This larval habitat choice results from a surprising ability and tendency to swim actively against all but the strongest oceanic currents. Among 11 common Great Barrier Reef families studied, larvae in the latter half of their larval phase could swim at speeds greater than the mean current speeds found around reef areas and thus influence their dispersal “on a magnitude similar to the dispersing
effect of oceanic currents” (Fisher 2005, p. 223). The biggest mystery in larval transport has been the means by which larvae traverse hundreds of kilometers to get from offshore spawning grounds to inshore nursery areas (i.e., anguillid eels, bonefishes, menhaden, scorpionfishes, croakers, bothid and pleuronectid flatfishes). Of obvious signifi cance is the spawning behavior of adults, especially their ability to place eggs in favorable locales. Spawning of many species on both the west and east coasts of North America occurs in winter when wind-driven, onshore currents are most common. Most marine eggs are buoyant and drift toward the surface. This places them in surface layers that are pushed shoreward by winds, either directly or as a result of Ekman transport, which involves wind-generated water movement that varies with depth.
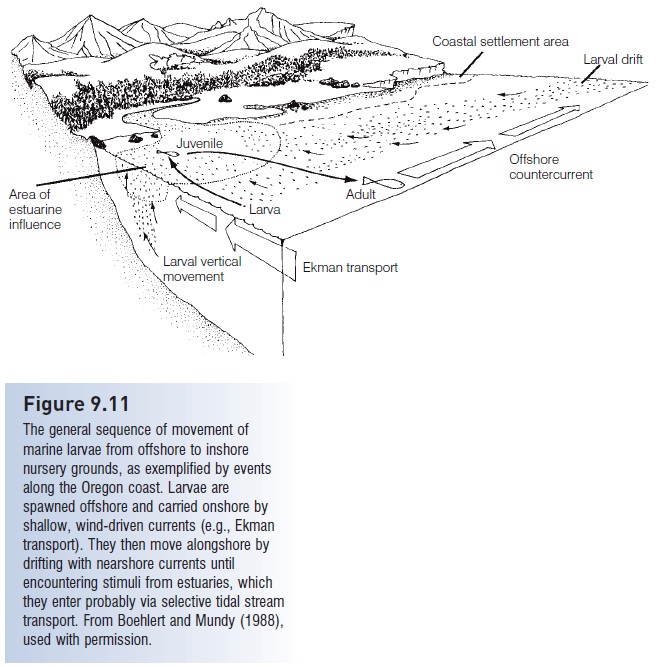
Figure 9.11
The general sequence of movement of marine larvae from offshore to inshore nursery grounds, as exemplified by events along the Oregon coast. Larvae are spawned offshore and carried onshore by shallow, wind-driven currents (e.g., Ekman transport). They then move alongshore by drifting with nearshore currents until encountering stimuli from estuaries, which they enter probably via selective tidal stream transport. From Boehlert and Mundy (1988), used with permission.
Some larvae may be carried by portions of major currents such as the Gulf Stream; the smaller water masses spin off from the main current and move shoreward as “warm core rings” (Hare et al. 2002). Active behavioral mechanisms must also influence distribution because larvae of different species that spawn in similar locales may wind up in different places. For example, mullet, Bluefish, and dolphinfish spawn offshore, but larvae of the first two species use inshore nurseries, whereas dolphinfish larvae remain offshore. Vertical movements by larvae into upper surface waters could aid in retaining them in water masses that were moving shoreward (Norcross & Shaw 1984), although active swimming by older larvae of such species as Bluefish is also likely (Hare & Cowen 1996).
Shoreward movement is complicated by the larva’s need to feed, so egg placement must also be in areas that are productive. Species that develop offshore often spawn in productive regions that are relatively stable, such as gyres, upwellings, fronts, or other circulating patterns that retain larvae where food availability remains high (e.g., pollock, Dover Sole). Some evidence suggests that coral reef fishes spawn at times and places that tend to retain larvae in local circulation patterns, which would promote their return to parental or nearby locales. Larval retention is at the heart of the controversy over whether coral reefs are selfrecruiting or are dependent on upstream larval sources, a dichotomy with direct application to the design and placement of marine reserves. Evidence is accumulating that at least for some locales and species, self-recruitment may be as common as widespread dispersal (e.g., Cowen et al. 2000; Hawkins et al. 2000; Taylor &Hellberg 2003). Given oceanographic features and currents and known larval behavior, between 37% and 80% of snapper larvae may self-recruit to Cuban waters (Pariset al. 2005). The pattern of currents and gyres around the Florida Keys would tend to aid retention of locally spawned larvae (Lee & Williams 1999). For isolated island regions such as Hawaii, larval retention might be insurance against dispersal into vast and uninhabitable oceanic regions (Norcross & Shaw 1984; Lobel 1989).
After the journey towards shore, larvae frequently accumulate along shorelines and at mouths of bays and estuaries, only to be found later inside thes regions. Such pulsed, directional movement against the general net flow of water out of an estuary may involveselective tidal stream transport. During such transport, small fish ride favorable currents and avoid unfavorable ones, usually by moving up into the water column on fl ood tides and down to the bottom during ebb tides (see Tidal patterns). This has been the suggested mechanism for movement into and retention within estuaries by young anguillid eels, herrings, shads, croakers, and plaice (Miller 1988).
Once in a nursery area, a fish must fight currents that would distribute it offshore or to less desirable inshore habitats. This becomes less of a problem as a juvenile fish grows larger and stronger and can actively choose locales or currents, but remains a significant constraint for small larvae of species such as Atlantic Herring (Clupea harengus). Herring spawn in the estuary itself, and larvae first move to upstream areas but later reside in downstream areas. This distribution is achieved by vertical movement with respect to different currents. In most estuaries, surface waters are relatively fresh and move downstream, bottom waters are relatively saline and move upstream, and intermediate depths exhibit no net directional movement. In the St. Lawrence River estuary of Canada, young herring larvae remain near the bottom and are consequently carried upriver, whereas older larvae tend to move up and down twice daily and hence hold position in a relatively confined area of the estuary (Fortier & Leggett 1983). Species that spawn in estuaries (e.g., various herrings, cods, flatfishes, wolffishes, sculpins, gobies) tend to have large, demersal eggs and brief larval stages, all characteristics that would minimize export from the habitat (Hempel 1979; Norcross & Shaw 1984).
Larvae would have to respond to environmental cues that informed them when they were approaching appropriate or inappropriate habitats. Postulated cues that larvae might use to discriminate between water masses include odor, salinity, oxygen, turbidity, pH, geomagnetism, turbulence, light, food availability, temperature, and current speed and direction. An intriguing possibility is that larvae of coral reef species hear sounds associated with reefs and consequently orient toward those sounds. Larval traps that incorporated underwater speakers that broadcast natural reef noises (snapping shrimp, fish vocalizations) attracted significantly more larvae and a greater diversity of larvae than silent traps, especially at night (Leis et al. 2003; Simpson et al. 2004; Tolimieri et al. 2004). These and other studies lead to the conclusion that “sound emanating from reefs at night is a useable sensory cue for fish larvae trying to find settlement habitat” (Leis & Lockett 2005, p. 715).
Similar evidence indicates that larvae are attracted to odors associated with reef areas (e.g., Atema et al. 2002). Clearly, multiple cues facilitate attraction to and settlement in appropriate habitat (Kingsford et al. 2002; Synthesis: what determines assemblage structure among coral reef fishes?).
Responses to any such cues are likely influenced by tidal, circadian, or lunar rhythms. Much discussion has focused on whether larvae can in fact detect minor differences in these factors among water masses and orient appropriately, and this remains an area of active research.
Many of our conclusions about larval transport remain conjectural because of a lack of confi rming data. In addition, alternative explanations that view larvae as passive, drifting particles that regulate little more than their buoy ancy readily explain certain aspects of their distribution. For example, rivers commonly enter estuaries and create ebb tides that are less saline than fl ood tides. Increased buoyancy in saltier water would tend to move drifting particles inshore with the fl ood, with no behavioral selection of water mass necessary (Miller 1988). Such purely passive drift cannot be totally dismissed in explanations of larval transport, although most evidence points to some type of behavioral regulation at most stages of development, at least once the egg stage is past.
Related Topics