Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Early life history
Gametogenesis - Eggs and sperm of Fishes
Eggs and sperm
Gametogenesis
Most fishes have paired gonads, although one member of the pair may be consistently larger than the other in some species or only one gonad may be functional. Hagfishes and lampreys are unique in that only one ovary develops, from the fusion of two primordia in lampreys and from the loss of one ovary in hagfishes. Unlike sharks and other vertebrates, testes and ovaries in jawless fishes and bony fishes develop from only the cortex of the peritoneal epithelium, not from both the cortex and medulla.Testes in immature males are typically reddish and take ona smooth texture and creamy-white coloration as the fish matures and spawning time approaches. The testes generally account for <5% of body weight (see below). Follicles within the testes produce the developing spermatozoa(=spermatogenesis) through a series of meiotic anddevelopmental transformations typical of vertebrates (spermatogonia,primary spermatocytes, secondary spermatocytes, spermatids, metamorphosis,spermatozoa) (Hoar1969; Hempel 1979; Gorbman 1983; Adkins-Regan 1987;Jamieson 1991).
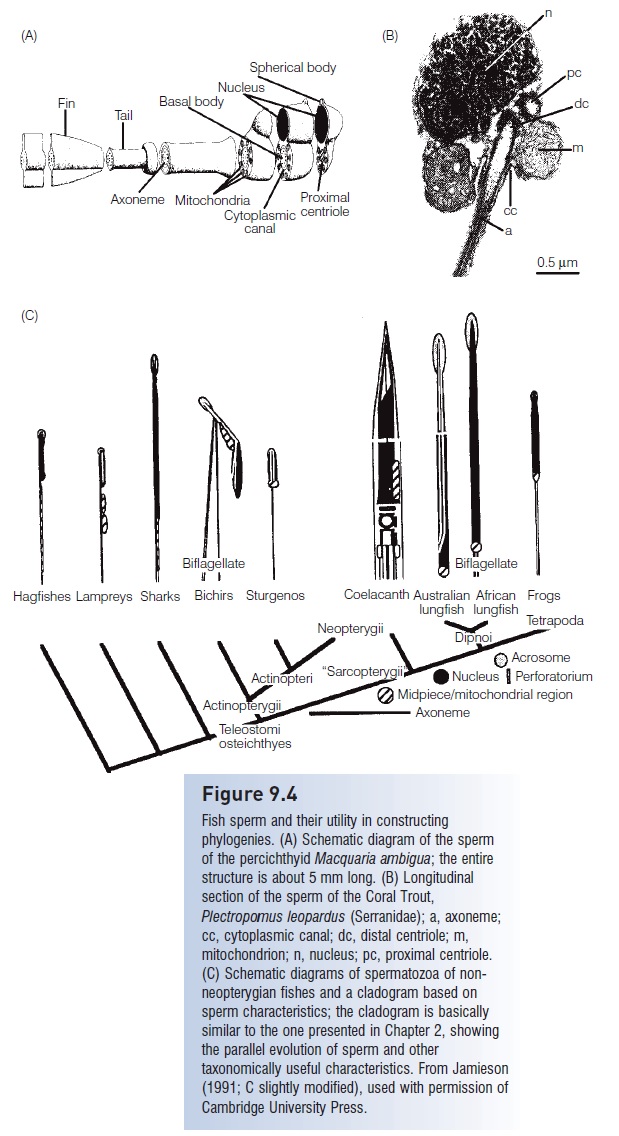
Figure 9.4
Fish sperm and their utility in constructing phylogenies. (A) Schematic diagram of the sperm of the percichthyid Macquaria ambigua; the entire structure is about 5 mm long. (B) Longitudinal section of the sperm of the Coral Trout, Plectropomus leopardus (Serranidae); a, axoneme;cc, cytoplasmic canal; dc, distal centriole; m, mitochondrion; n, nucleus; pc, proximal centriole. (C) Schematic diagrams of spermatozoa of nonneopterygian fishes and a cladogram based on sperm characteristics; the cladogram is basically similar to the one presented, showing the parallel evolution of sperm and other taxonomically useful characteristics. From Jamieson (1991; C slightly modified), used with permission of Cambridge University Press.
Fish sperm vary in size, shape, number of flagella (noneto two), and presence or absence of acrosomes and other structures. Fish sperm are highly diagnostic of higher taxa and of some species (Jamieson 1991) (Fig. 9.4). Sperm heads range in length from about 2 mm (Bowfin, burbot,medaka) to 70 mm (Australian Lungfish). The caplikeacrosomeat the anterior end of most primitive fish sperm is lost in practically all neopterygian fishes (gars, Bowfin, teleost’s).
Two African families of osteoglossomorph fishes, theMormyridae (elephant fishes or mormyrs) and Gymnarchidae,lack a flagellum. Their sperm may move by some formofameboid motion. Typical ejaculates during spawning contain millions of sperm. Sperm is released in seminal fluid in species with external fertilization, or in packets called spermatophores in internal fertilizers. It is commonly stated that males produce an excess of sperm and consequently male reproductive success is limited more by access to females than by ability to produce gametes (theopposite is considered limiting in females). However, under circumstances where males mate daily over a prolonged breeding season, sperm depletion can occur and mating may in fact be delayed until sperm stores are replenished(e.g., Nakatsaru & Kramer 1982; Jamieson 1991; see alsoShapiro et al. 1994).
Oogenesis, the development of eggs, occurs within the ovary and also progresses through various stages involvingoogonia, primary and secondary oocytes, and finallyova or eggs (Wallace & Selman 1981) (Fig. 9.5). Oogones develop from primordial sex cells in the germinal epithelium of the ovary wall. Proteinaceous yolk granules are deposited
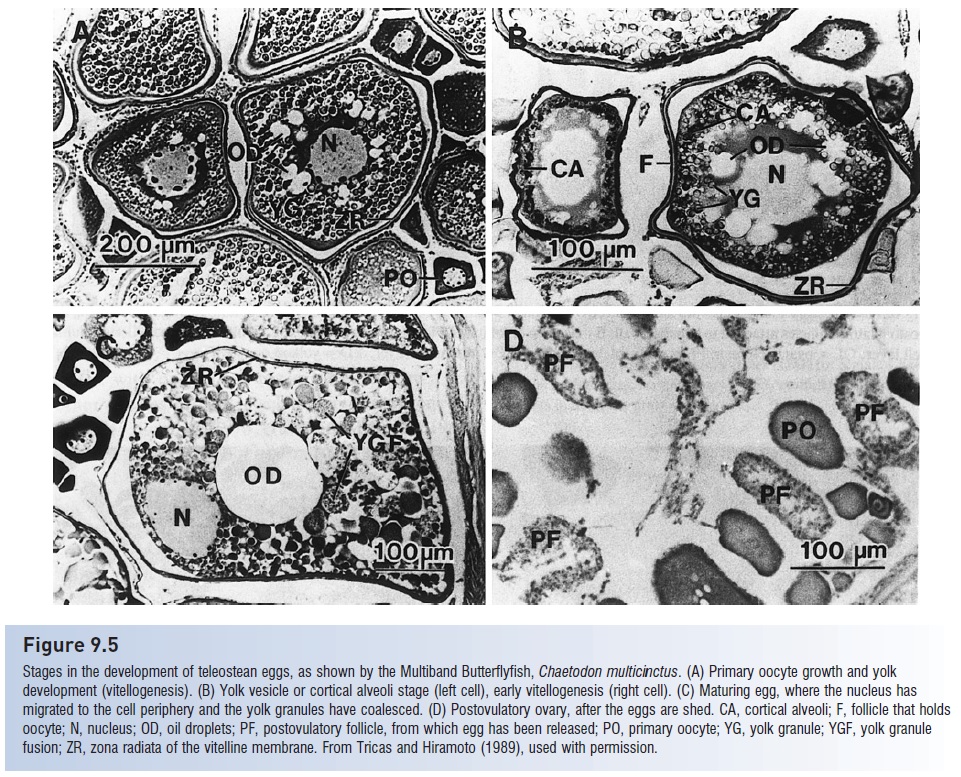
Figure 9.5
Stages in the development of teleost an eggs, as shown by the Multiband Butterflyfish, Chaetodon multicinctus. (A) Primary oocyte growth and yolk development (vitellogenesis). (B) Yolk vesicle or cortical alveoli stage (left cell), early vitellogenesis (right cell). (C) Maturing egg, where the nucleus has migrated to the cell periphery and the yolk granules have coalesced. (D) Postovulatory ovary, after the eggs are shed. CA, cortical alveoli; F, follicle that holds oocyte; N, nucleus; OD, oil droplets; PF, postovulatory follicle, from which egg has been released; PO, primary oocyte; YG, yolk granule; YGF, yolk granule fusion; ZR, zona radiata of the vitelline membrane. From Tricas and Hiram to (1989), used with permission.
Oil droplets are incorporated in the yolk. Ripe eggs pass from the ovary through the oviduct, which is a continuation of the ovarian tissue, to the outside via the cloaca. In elasmobranchs,no direct connection between ovary and oviduct exists and hence eggs pass through the peritoneal cavity on their way to the oviduct. In several osteoglossomorphbonytongues, a loach (Misgurnus), anguillids, salmonids, and galaxiids, the oviduct is greatly reduced or absent and eggs enter the body cavity prior to being shed (Blaxter1969; Hempel 1979; Wootton 1990). Females that have spawned are termed spent; their ovaries are bloody andcontain residual eggs which are resorbed by the ovary. Egg resorption is a general process. Usually, most of the ripe eggs in an ovary are spawned while a small proportion is resorbed and the proteins, fats, and minerals contained in them are reused by the female for maintenance, growth, or production of more eggs. The number resorbed varies greatly among and within species, depending on fish size, number of previous spawning that season, and energy state of the female.
Fecundity, the number of eggs released by a female during a spawning bout or breeding cycle, varies from oneto two in some sharks to tens of millions in the Tarpon,Megalops atlanticus, and European Ling, Molva molva, to300 x106 in the Giant Ocean Sunfish, Mola mola; seasonal and lifetime fecundities can also be calculated. Most larger, temperate marine fishes produce tens of thousands to millions of eggs at a time.
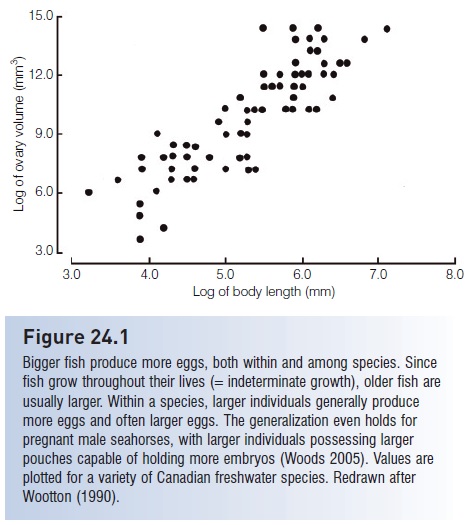
Fecundity generally decreases with increasing egg size and with increasing parental care, but increases with body size in an individual. Mouth-brooders such as sea catfishes and some cichlids produce only about100 eggs at a time, and live-bearers such as the Four-eyed Fish, Anableps, contain about a dozen embryos. The relationship between egg number and body size is usually proportional to the mass of the female, reflecting the volume of a female’s body that can carry the eggs. Hence egg number generally increases in relation to the cube, fourth, or fifth power of the length of the female (see Fig. 24.1).In addition to producing more eggs, larger females of many species produce bigger, better eggs that result in higher larval survival (e.g., in salmons, cod, haddock, Striped Bass, flounder) (Trippel 1995).
Exceptions show the premium placed on assuring survival of young rather than just on producing large numbers of eggs. In mouth-brooding cichlids, fecundity increases in relation to the square of the length of the female because mouth size increases only linearly with increasing body length (Breder & Rosen 1966; Hempel1979; Lowe-McConnell 1987). Because of resorption, fecundity estimates based on counts of ripe eggs may not necessarily indicate true fertility, which is the number of viable offspring produced. Fecundity estimates for livebearing fishes are further complicated by the consumptionof eggs by developing embryos, a form of maternal provisioning (see below).
Certain generalizations apply to fish eggs, with a strong correlation between habitat and the characteristics of fertilized eggs. “Most marine fishes, regardless of systematic affinities, demersal or pelagic habits, coastal or oceanicdistribution, tropical or boreal ranges, spawn pelagic eggs that are fertilized externally and float individually near the surface of the sea” (Kendall et al. 1984, p. 11). Among pelagic spawners, eggs are generally spherical in shape and have a single oil globule. Their diameters range from about 0.5 mm (Vinciguerria, Photichthyidae) to 5.5 mm (moray eels), with a modal size of about 1 mm.
This remarkable convergence among phylogenetically widespread taxa suggests a common, adaptive set of solutions to the selection pressures encountered by eggs that disperse passively in a near-surface environment and that contain an embryo dependent on yolk supplies for nutrition.
Most freshwater fishes and some coastal marine species diverge from this pattern and produce demersal eggs thatare laid on the bottom. Many spawn in nests and engage in some form of parental care. Demersal eggs tend to be relatively large, up to 7 or 8 mm in salmon, anarhichadid wolf fishes, and zoarcid eelpouts. The largest teleost an eggs are produced by mouth-brooding marine catfishes and range from 14 to 26 mm. Shark eggs are generally larger than osteichthyan eggs, whereas the largest bony fish eggs are produced by the Coelacanth, Latimeria chalumnae, with a diameter of 9 cm. Demersal eggs often have thick chorions and special coatings that may provide mechanical protection (ictal rid catfishes, North American minnows, killifishes, freshwater perch, blennies) (Boehlert 1984; Matarese &Sand knop 1984).
Departures from spherical shape are found in the elongate eggs of some cusk-eels, anchovies, minnows, cichlids,parrotfishes, and gobies. Congrogadid eel blennies have strangely cross-shaped eggs, and eggs of some darters are deeply indented and appear almost heart-shaped. Although usually smooth, the outer vitelline membrane of the egg, termed the chorion, may be sculptured (lizardfishes, deep-seahatchet fishes, mullets, some flounders), or have filaments, stalks, or spines (myctophiform lantern fishes and many atherinomorphs such as killifishes, flying fishes, tops melts, sauries, and halfbeaks). Filaments often help eggs attach to other eggs or to structures such as seaweeds, as in flying fishes. Variation in other egg structures can help in species or family identification. The degree of segmentation and
Pigmented yolks produce colorful eggs in gar, catfishes, and salmon. The occurrence, number, and location of oil globules in the yolk differ among species. Oil globules may serve as nutrition for embryos, as fl otation mechanisms, and, when pigmented with melanin, may help protect sensitive developing structures from harmful radiation (F. D. Martin, pers. comm.). Oil globules in some species go through highly predictable patterns of movement (Malloy & Martin 1982). Eggs also vary among species in terms of the space between the chorion and the yolk, termed the perivitelline space or “around the yolk” space.
An interesting aspect of reproduction is the effort and energy that different species and individuals expend, which often correlates with the life history pattern a species has evolved (see Life histories and reproductive ecology). Reproductive effort includes food intake and its transfer to the gonads, as well as energy expenditure in somatic versus gonadal growth. In females, oocyte maturation involves mobilization of first lipids and then proteins from other parts of the body, such as fat deposits and body muscle, to the ovary. This maturation is also accompanied by as much as a 10-fold increase in oxygen consumption by the ovary until the final stage of oogenesis, when ovarian mass increases via water accumulation. True costs of reproduction must also account for energy expended during reproductive migrations, courtship, spawning, and internal brooding and other forms of parental care, among other factors. Energy expended in nongonadal growth can be substantial. For example, migratory female sturgeon and salmon use 80–90% of their body fat reserves during reproduction, much of which is expended during migration to the spawning grounds (Kamler 1991).
The difficulty of estimating reproductive effort accurately has led to the development of a variety of indices. Some indices depict characteristics of an individual at a given point in time (instantaneous measures), others over the reproductive life of an individual (cumulative measures). One popular and simple instantaneous measure is the GSI, variously called the gonadosomatic, or gonadalsomatic, or gonosomatic index, a calculation of the percentage of the body mass of an animal devoted to gonadal material. The GSI can be calculated as gonad weight/total body weight, or as (gonad weight/body weight) – gonad weight (and sometimes minus gut weight). Values range greatly among species: cichlids <5%, darters 11–23%, pupfishes 2–14%, American plaice 5–20%, sticklebacks 20%, salmonids 20–30%, and European eels 47%. The GSI of ripe females is a relatively accurate portrayal of effort fortotal spawners that spawn only once in a breeding season or lifetime, but it underestimates effort in repeat, batch, or serial spawners because only a fraction of the eggs or oocytes a female will produce are present at any moment (Heins & Rabito 1986).
GSIs in males are generally much smaller than for females, reflecting a lower effort directly expended in reproduction in males of many species. GSIs in male sticklebacks are 2% compared to 20% for females, and only 0.2% in male tilapia. Female Pike, Esox lucius, allocate 6– 18 times more energy to ovaries than males do to testes. Intraspecific variation in male GSI can reflect differences in reproductive tactics. In Bluegill Sunfish, Lepomis macrochirus, some males guard nests and attract females with which they spawn; other males lurk at the periphery of the nests and sneak into the territory to join a spawning nest-guarder. GSIs for territorial males run at about 1%, but for sneakers are 4.5%. GSIs in male Bluehead Wrasses vary between 3% and 5%, the larger value characterizing males that previously engaged in group spawning, where sperm competition among males is likely (Gross 1982; Shapiro et al. 1994).
Measuring the GSI during different parts of the year can indicate ovarian or testicular cycles and spawning periodicities. Multimodal values would indicate protracted spawning seasons, whereas a single maximum would indicate a more defined spawning season. Not surprisingly, GSI values generally reach their maxima just before spawning. GSI calculations have been modified and improved to account for differences in body size among females and for females that do not shed all eggs at once (DeMartini & Fountain 1981; Erickson et al. 1985).
Related Topics