Chapter: Medical Surgical Nursing: Assessment of Respiratory Function
Function of the Respiratory System
FUNCTION OF THE RESPIRATORY SYSTEM
The cells of the body derive the energy
they need from the oxi-dation of carbohydrates, fats, and proteins. As with any
type of combustion, this process requires oxygen. Certain vital tissues, such
as those of the brain and the heart, cannot survive for long without a
continuing supply of oxygen. However, as a result of oxidation in the body
tissues, carbon dioxide is produced and must be removed from the cells to
prevent the buildup of acid waste products. The respiratory system performs
this function by facilitating life-sustaining processes such as oxygen
transport, res-piration and ventilation, and gas exchange.
Oxygen Transport
Oxygen
is supplied to, and carbon dioxide is removed from, cells by way of the
circulating blood. Cells are in close contact with capillaries, whose thin
walls permit easy passage or exchange of oxygen and carbon dioxide. Oxygen
diffuses from the capillary through the capillary wall to the interstitial
fluid. At this point, it diffuses through the membrane of tissue cells, where
it is used by mitochondria for cellular respiration. The movement of carbon
dioxide occurs by diffusion in the opposite direction—from cell to blood.
Respiration
After
these tissue capillary exchanges, blood enters the systemic veins (where it is
called venous blood) and travels to the pul-monary circulation. The oxygen
concentration in blood within the capillaries of the lungs is lower than in the
lungs’ air sacs (alve-oli). Because of this concentration gradient, oxygen
diffuses from the alveoli to the blood. Carbon dioxide, which has a higher
con-centration in the blood than in the alveoli, diffuses from the blood into
the alveoli. Movement of air in and out of the airways (ventilation)
continually replenishes the oxygen and removes the carbon dioxide from the
airways in the lung. This whole process of gas exchange between the atmospheric
air and the blood and between the blood and cells of the body is called respiration.
Ventilation
During
inspiration, air flows from the environment into the tra-chea, bronchi,
bronchioles, and alveoli. During expiration, alve-olar gas travels the same
route in reverse.
Physical
factors that govern air flow in and out of the lungs are collectively referred
to as the mechanics of ventilation and include air pressure variances,
resistance to air flow, and lung compliance.
AIR PRESSURE VARIANCES
Air
flows from a region of higher pressure to a region of lower pressure. During
inspiration, movement of the diaphragm and other muscles of respiration
enlarges the thoracic cavity and thereby lowers the pressure inside the thorax
to a level below that of atmospheric pressure. As a result, air is drawn
through the tra-chea and bronchi into the alveoli.
During
normal expiration, the diaphragm relaxes and the lungs recoil, resulting in a
decrease in the size of the thoracic cav-ity. The alveolar pressure then
exceeds atmospheric pressure, and air flows from the lungs into the atmosphere.
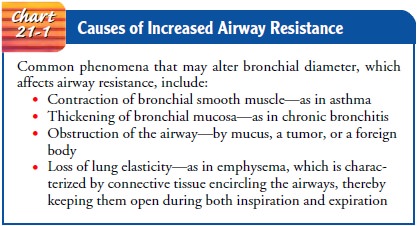
AIRWAY RESISTANCE
Resistance
is determined chiefly by the radius or size of the airway through which the air
is flowing. Any process that changes the bronchial diameter or width affects
airway resistance and alters the rate of air flow for a given pressure gradient
during respiration (Chart 21-1). With increased resistance, greater-than-normal
res-piratory effort is required by the patient to achieve normal levels of
ventilation.
COMPLIANCE
The
pressure gradient between the thoracic cavity and the atmos-phere causes air to
flow in and out of the lungs. When pressure changes are applied in the normal
lung, there is a proportional change in the lung volume. A measure of the
elasticity, expand-ability, and distensibility of the lungs and thoracic structures
is called compliance. Factors that determine lung compliance are the surface
tension of the alveoli (normally low with the presence of surfactant) and the
connective tissue (ie, collagen and elastin) of the lungs.
Compliance
is determined by examining the volume–pressure relationship in the lungs and
the thorax. In normal compliance (1.0 L/cm H2O),
the lungs and thorax easily stretch and distend when pressure is applied. High
or increased compliance occurs when the lungs have lost their elasticity and
the thorax is overdistended (ie, in emphysema). When the lungs and thorax are
“stiff,” there is low or decreased compliance. Conditions as-sociated with this
include pneumothorax, hemothorax, pleural effusion, pulmonary edema,
atelectasis, pulmonary fibrosis, and acute respiratory distress syndrome
(ARDS), all of which are discussed in later. Measurement of com-pliance is one
method used to assess the progression and im-provement in ARDS. Lungs with
decreased compliance require greater-than-normal energy expenditure to achieve
normal lev-els of ventilation. Compliance is usually measured under static
conditions.
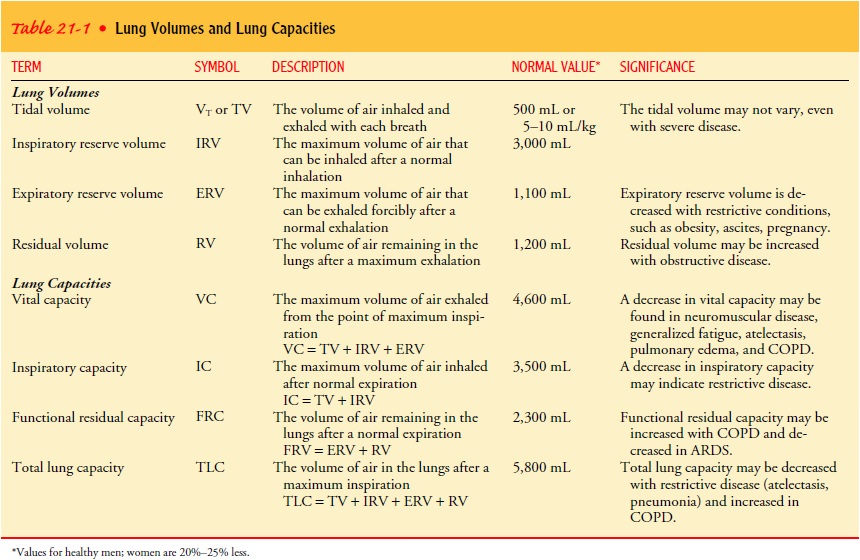
Lung Volumes and Capacities
Lung
function, which reflects the mechanics of ventilation, is viewed in terms of
lung volumes and lung capacities. Lung vol-umes are categorized as tidal
volume, inspiratory reserve volume, expiratory reserve volume, and residual
volume. Lung capacity is evaluated in terms of vital capacity, inspiratory
capacity, func-tional residual capacity, and total lung capacity. These terms
are described in Table 21-1.
Diffusion and Perfusion
Diffusion is
the process by which oxygen and carbon dioxide areexchanged at the air–blood
interface. The alveolar–capillary membrane is ideal for diffusion because of
its large surface area and thin membrane. In the normal healthy adult, oxygen
and car-bon dioxide travel across the alveolar–capillary membrane with-out
difficulty as a result of differences in gas concentrations in the alveoli and
capillaries.
Pulmonary perfusion is
the actual blood flow through thepulmonary circulation. The blood is pumped
into the lungs by the right ventricle through the pulmonary artery. The
pulmonary artery divides into the right and left branches to supply both lungs.
These two branches divide further to supply all parts of each lung. Normally
about 2% of the blood pumped by the right ventricle does not perfuse the
alveolar capillaries. This shunted blood drains into the left side of the heart
without participating in alveolar gas exchange.
The
pulmonary circulation is considered a low-pressure sys-tem because the systolic
blood pressure in the pulmonary artery is 20 to 30 mm Hg and the diastolic
pressure is 5 to 15 mm Hg. Because of these low pressures, the pulmonary
vasculature nor-mally can vary its capacity to accommodate the blood flow it
re-ceives. When a person is in an upright position, however, the pulmonary
artery pressure is not great enough to supply blood to the apex of the lung
against the force of gravity. Thus, when a per-son is upright, the lung may be
considered to be divided into three sections: an upper part with poor blood
supply, a lower part with maximal blood supply, and a section in between the
two with an intermediate supply of blood. When a person lying down turns to one
side, more blood passes to the dependent lung.
Perfusion
also is influenced by alveolar pressure. The pul-monary capillaries are
sandwiched between adjacent alveoli. If the alveolar pressure is sufficiently
high, the capillaries will be squeezed. Depending on the pressure, some
capillaries completely collapse, whereas others narrow.
Pulmonary artery pressure, gravity, and alveolar pressure de-termine the patterns of perfusion. In lung disease these factors vary, and the perfusion of the lung may become very abnormal.
Ventilation and Perfusion Balance and Imbalance
Ventilation is the flow of gas in and
out of the lungs, and perfu-sion is the filling of the pulmonary capillaries
with blood. Ade-quate gas exchange depends on an adequate ventilation–perfusion
ratio. In different areas of the lung, the ratio varies.
Alterations in perfusion may occur with
a change in the pul-monary artery pressure, alveolar pressure, and gravity.
Airway blockages, local changes in compliance, and gravity may alter
ventilation.˙ ˙
A ventilation–perfusion V/Q imbalance occurs from inade-quate ventilation, inadequate perfusion, or both. There are four˙ possible V/Q states in the lung: normal V/Q ratio, low V/Q ratio (shunt), high V/Q ratio (dead space), and absence of ventilation and perfusion (silent unit) (Chart 21-2).
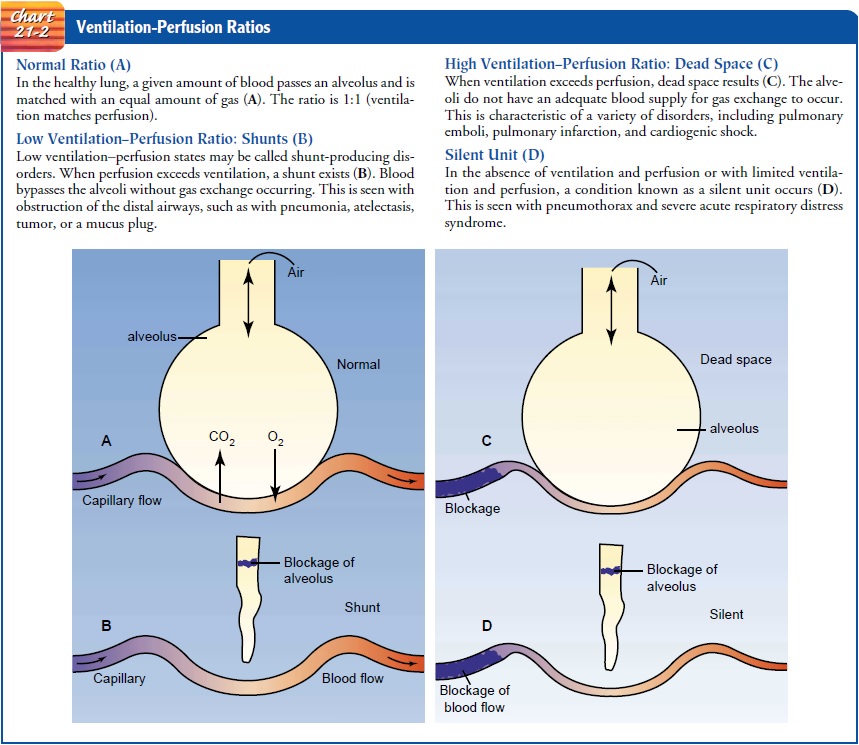
Ventilation
and perfusion imbalance causes shunting of blood, resulting in hypoxia (low cellular oxygen level). Shunting
appears to be the main cause of hypoxia after thoracic or abdom-inal surgery
and most types of respiratory failure. Severe hypoxia results when the amount
of shunting exceeds 20%. Supplemental oxygen may eliminate hypoxia, depending
on the type of V/Q imbalance.
Gas Exchange
The
air we breathe is a gaseous mixture consisting mainly of ni-trogen (78.62%) and
oxygen (20.84%), with traces of carbon dioxide (0.04%), water vapor (0.05%),
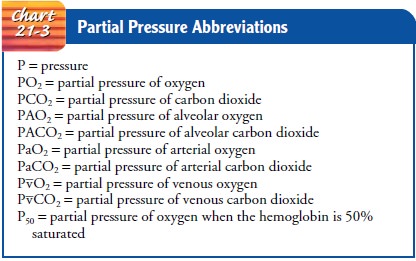
helium, and argon. The atmospheric pressure at sea level is about 760 mm Hg.
Partial pressure is the pressure exerted by each type of gas in a mixture of
gases. The partial pressure of a gas is proportional to the concen-tration of
that gas in the mixture. The total pressure exerted by the gaseous mixture is
equal to the sum of the partial pressures.
PARTIAL PRESSURE OF GASES
Based on these facts, the partial pressures of nitrogen and oxygen can be calculated. The partial pressure of nitrogen is 79% of 760 (0.79 × 760), or 600 mm Hg; that of oxygen is 21% of 760 (0.21 760), or 160 mm Hg. Chart 21-3 spells out terms and abbre-viations related to partial pressure of gases.
Once
the air enters the trachea, it becomes fully saturated with water vapor, which
displaces some of the gases so that the air pres-sure within the lung remains
equal to the air pressure outside
(760
mm Hg). Water vapor exerts a pressure of 47 mm Hg when it fully saturates a
mixture of gases at the body temperature of 37°C
(98.6°F).
Nitrogen and oxygen are responsible for the re-maining 713 mm Hg (760 − 47) pressure. Once this mixture en-ters the alveoli, it is
further diluted by carbon dioxide. In the alveoli, the water vapor continues to
exert a pressure of 47 mm Hg. The remaining 713 mm Hg pressure is now exerted
as fol-lows: nitrogen, 569 mm Hg (74.9%); oxygen, 104 mm Hg (13.6%); and carbon
dioxide, 40 mm Hg (5.3%).
PARTIAL PRESSURE IN GAS EXCHANGE
When
a gas is exposed to a liquid, the gas dissolves in the liquid until an
equilibrium is reached. The dissolved gas also exerts a partial pressure. At
equilibrium, the partial pressure of the gas in the liquid is the same as the
partial pressure of the gas in the gaseous mixture. Oxygenation of venous blood
in the lung illus-trates this point. In the lung, venous blood and alveolar
oxygen are separated by a very thin alveolar membrane. Oxygen diffuses across
this membrane to dissolve in the blood until the partial pressure of oxygen in
the blood is the same as that in the alveoli (104 mm Hg). However, because
carbon dioxide is a byproduct of oxidation in the cells, venous blood contains
carbon dioxide at a higher partial pressure than that in the alveolar gas. In
the lung, carbon dioxide diffuses out of venous blood into the alveolar gas. At
equilibrium, the partial pressure of carbon dioxide in the blood and in
alveolar gas is the same (40 mm Hg). The changes in partial pressure are shown
in Figure 21-5.
EFFECTS OF PRESSURE ON OXYGEN TRANSPORT
Oxygen and carbon dioxide are transported simultaneously dis-solved in blood or combined with some of the elements of blood. Oxygen is carried in the blood in two forms: first as physically dissolved oxygen in the plasma, and second in combination with the hemoglobin of the red blood cells. Each 100 mL of normal arte-rial blood carries 0.3 mL of oxygen physically dissolved in the plasma and 20 mL of oxygen in combination with hemoglobin.
Large amounts of oxygen can be transported in the blood because it
combines easily with hemoglobin to form oxyhemoglobin:

The volume of oxygen physically
dissolved in the plasma varies directly with the partial pressure of oxygen in
the arteries (PaO2). The higher the PaO2, the greater the amount of oxygen dissolved. For
example, at a PaO2 of 10 mm Hg, 0.03 mL of oxy-gen is
dissolved in 100 mL of plasma. At 20 mm Hg, twice this amount is dissolved in
plasma, and at 100 mm Hg, 10 times this amount is dissolved. Therefore, the
amount of dissolved oxygen is directly proportional to the partial pressure,
regardless of how high the oxygen pressure rises.
The
amount of oxygen that combines with hemoglobin also depends on PaO2,
but only up to a PaO2 of about
150 mm Hg. When the PaO2 is 150 mm
Hg, hemoglobin is 100% saturated and will not combine with any additional
oxygen. When hemoglobin is 100% saturated, 1 g of hemoglobin will combine with
1.34 mL of oxygen. Therefore, in a person with 14 g/dL of hemoglobin, each 100
mL of blood will contain about 19 mL of oxygen associ-ated with hemoglobin. If
the PaO2 is less than 150 mm Hg, the
percentage of hemoglobin saturated with oxygen is lower. For ex-ample, at a PaO2
of 100 mm Hg (normal value), saturation is 97%; at a PaO2
of 40 mm Hg, saturation is 70%.
OXYHEMOGLOBIN DISSOCIATION CURVE
The oxyhemoglobin dissociation curve (Chart 21-4) shows the relationship between the partial pressure of oxygen (PaO2) and the percentage of saturation of oxygen (SaO2). The percentage of saturation can be affected by the following factors: carbon diox-ide, hydrogen ion concentration, temperature, and 2,3-diphos-phoglycerate.
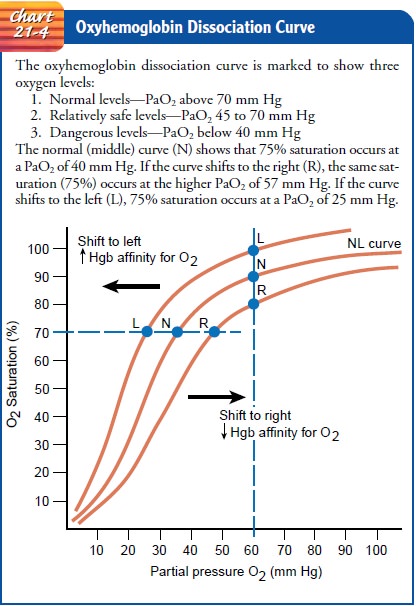
A rise in these factors shifts the curve to the right so that more oxygen is
then released to the tissues at the same PaO2.
A reduction in these factors causes the curve to shift to the left, making the
bond between oxygen and hemoglobin stronger, so that less oxygen is given up to
the tissues at the same PaO2. The
unusual shape of the oxyhemoglobin dissociation curve is a dis-tinct advantage
to the patient for two reasons:
·
If
the arterial PO2 decreases from 100 to 80 mm Hg as a
result of lung disease or heart disease, the hemoglobin of the arterial blood
remains almost maximally saturated (94%) and the tissues will not suffer from
hypoxia.
·
When the arterial blood passes into
tissue capillaries and is exposed to the tissue tension of oxygen (about 40 mm
Hg), hemoglobin gives up large quantities of oxygen for use by the tissues.
Clinical Significance.
The normal value of PaO2 is 80 to 100 mm
Hg (95% to 98% saturation). With this level of oxygenation, there is a 15%
margin of excess oxygen available to the tissues. With a normal hemoglobin
level of 15 mg/dL and a PaO2 level of 40 mm Hg (oxygen saturation 75%), there
is adequate oxygen available for the tissues but no reserve for physiologic
stresses that increase tissue oxygen demand. When a serious incident oc-curs
(eg, bronchospasm, aspiration, hypotension, or cardiac dys-rhythmias) that
reduces the intake of oxygen from the lungs, tissue hypoxia will result.
An important consideration in the transport of oxygen is car-diac output, which determines the amount of oxygen delivered to the body and which affects lung and tissue perfusion. If the cardiac output is normal (5 L/min), the amount of oxygen delivered to the body per minute is normal.
If cardiac output falls, the amount of oxygen delivered
to the tissues also falls. Under normal conditions, most of the oxygen
delivered to the body is not used. In fact, only 250 mL of oxygen is used per
minute. Under normal conditions, this is approximately 25% of available oxygen.
The rest of the oxy-gen returns to the right side of the heart, and the PaO2
of venous blood drops from 80 to 100 mm Hg to about 40 mm Hg.
Carbon Dioxide Transport
At
the same time that oxygen diffuses from the blood into the tis-sues, carbon
dioxide diffuses in the opposite direction (ie, from tis-sue cells to blood)
and is transported to the lungs for excretion. The amount of carbon dioxide in
transit is one of the major determi-nants of the acid–base balance of the body.
Normally, only 6% of the venous carbon dioxide is removed, and enough remains
in the arterial blood to exert a pressure of 40 mm Hg. Most of the carbon
dioxide (90%) enters the red blood cells; the small portion (5%) that remains
dissolved in the plasma (PCO2) is the
critical factor that determines carbon dioxide movement in or out of the blood.
In
summary, the many processes involved in respiratory gas transport do not occur
in intermittent stages; rather, they are rapid, simultaneous, and continuous.
Neurologic Control of Ventilation
Resting
respiration is the result of cyclical excitation of the res-piratory muscles by
the phrenic nerve. The rhythm of breathing is controlled by respiratory centers
in the brain. The inspiratory and expiratory centers in the medulla oblongata
and pons con-trol the rate and depth of ventilation to meet the body’s
metabolic demands.
The
apneustic center in the lower pons stimulates the inspira-tory medullary center
to promote deep, prolonged inspirations. The pneumotaxic center in the upper
pons is thought to control the pattern of respirations.
Several
groups of receptor sites assist in the brain’s control of respiratory function.
The central chemoreceptors are located in the medulla and respond to chemical
changes in the cerebrospinal fluid, which result from chemical changes in the
blood. These re-ceptors respond to an increase or decrease in the pH and convey
a message to the lungs to change the depth and then the rate of ventilation to
correct the imbalance. The peripheral chemore-ceptors are located in the aortic
arch and the carotid arteries and respond first to changes in PaO2,
then to PaCO2 and pH. The Hering–Breuer reflex is
activated by stretch receptors in the alve-oli. When the lungs are distended,
inspiration is inhibited; as a result, the lungs do not become overdistended.
In addition, pro-prioceptors in the muscles and joints respond to body
move-ments, such as exercise, causing an increase in ventilation. Thus,
range-of-motion exercises in an immobile patient stimulate breathing.
Baroreceptors, also located in the aortic and carotid bodies, respond to an
increase or decrease in arterial blood pres-sure and cause reflex
hypoventilation or hyperventilation.
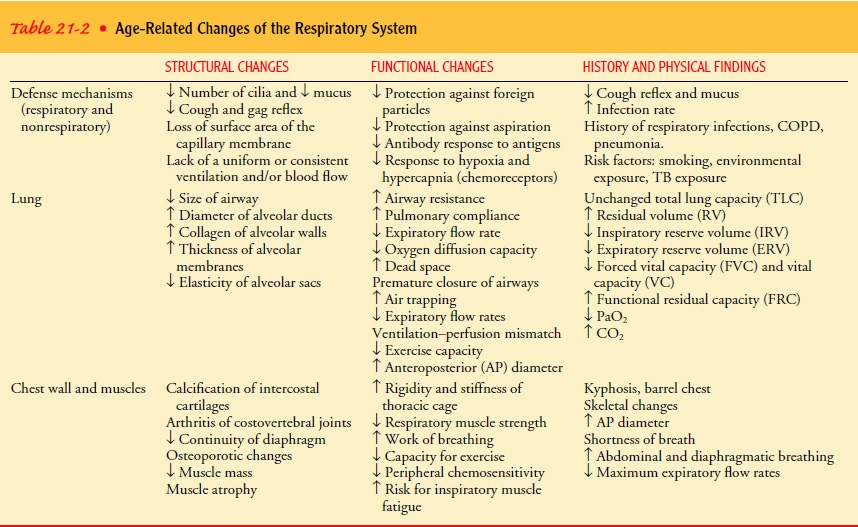
Gerontologic Considerations
A
gradual decline in respiratory function begins in early to middle adulthood and
affects the structure and function of the respiratory system. The vital
capacity of the lungs and respiratory muscle strength peak between ages 20 and
25 and decrease thereafter. With aging (40 years and older), changes occur in
the alveoli that reduce the surface area available for the exchange of oxygen
and carbon dioxide. At approximately age 50, the alveoli begin to lose
elasticity. A decrease in vital capacity occurs with loss of chest wall
mobility, thus restricting the tidal flow of air. The amount of res-piratory
dead space increases with age. These changes result in a decreased diffusion
capacity for oxygen with age, producing lower oxygen levels in the arterial
circulation. Elderly people have a decreased ability to move air rapidly in and
out of the lungs. Geron-tologic changes in the respiratory system are
summarized in Table 21-2. Despite these changes, in the absence of chronic pulmonary
disease, elderly people are able to carry out activities of daily living, but
they may have decreased tolerance for and require additional rest after
prolonged or vigorous activity.
Related Topics