Chapter: Mechanical and Electrical : Power Plant Engineering : Neclear Power Plants
Fission Energy, Chain Reaction, Fusion Energy
Fission Energy, Chain Reaction, Fusion Energy
(a)Fission Energy (b) Chain Reaction (c) Fusion Energy
A nuclear power plant is similar to a conventional steam
power plant except how that energy is evolved. The heat is produced in the
nuclear power plant by fission, whereas in steam and gas turbine plants, the
heat is produced by combustion in the furnace. The nuclear reactor acts as a
furnace where nuclear energy is evolved by splitting or fissioning of the
nucleus of fissionable material like Uranium U-235. It is claimed that 1 kg
U-235 can produce as much heat energy that can be produced by burning 4500 tones
of high grade coal or 1700 tons of oil.
Fission
energy
Nuclear energy is
divided from splitting
(or) fissioning of
the nucleus of
fissionable material like Uranium U-235. Uranium has several isotopes
(Isotopes are atoms of the same element having different atomic masses) such as
U-234, U-235 and U-238. Of the several isotopes, U-235 is the most unstable
isotope, which is easily fissionable and hence used as fuel in an atomic
reactor.
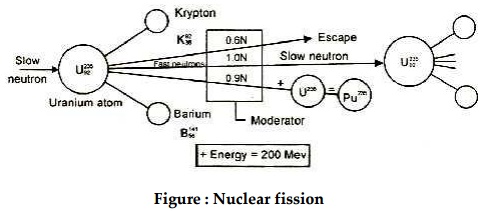
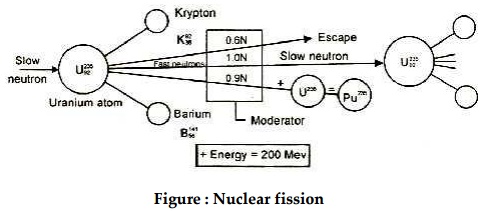
When a neutron enters the nucleus of an unstable U-235,
the nucleus splits into two equal fragments (Krypton and Barium) and also
releases 2.5 fast moving neutrons with a velocity of 1.5×107 m/sec
and along with this produces a large amount of energy, nearly 200 million
electro-volts. This is called nuclear fission.
1.
Chain reaction
The neutrons released during fission are very fast and can
be made to initiate the fission of other nuclei of U-235, thus causing a chain
reaction. When a large number of fission occurs, enormous amount of heat is
generated, which is used to produce steam.
The chain reaction under controlled conditions can release
extremely large amount of energy causing ‚atomic explosion‛
Energy released in chain reaction,
according to Einstein law is
E = mc2
Where E = Energy liberated (J) m= Mass (kg)
c = Velocity of light (3 × 108
m/sec).
Out of 2.5 neutrons released in fission of each nucleus of
U-235, one neutron is used to sustain the chain reaction, about 0.9 neutron is
captured by U-238, which gets converted into fissionable material Pu-239 and
about 0.6 neutron is partially absorbed by control rod materials, coolant and
moderator.
If thorium is used in the reactor
core, it gets converted to fissionable material U-233.
Thorium 232 + Neutron ® U-233
Pr-239 and U-233 so produced are fissionable materials are
called secondary fuels. They can be used as nuclear fuels. U-238 and Th-232 are
called fertile materials.
2.
Fusion energy
Energy is produced in the sun and
stars by continuous fusion reactions in which four nuclei of hydrogen fuse in a
series of reactions involving other particles that continually appear and
disappear in the course of the reaction, such as He3, nitrogen,
carbon, and other nuclei, but culminating in one nucleus of helium of two
positrons.
®
41 H 1 +2+1 e 4 0
® 2 He
To cause
fusion, it is necessary to accelerate the positively charged unclei to high
kinetic energies, in order to overcome electrical repulsive forces, by raising
their temperature to hundreds of millions of degrees resulting in plasma. The
plasma must be prevented from contacting the walls of the container, and must
be confined for a period of time (of the order of a second) at a minimum
density. Fusion reactions are called thermonuclear because very high
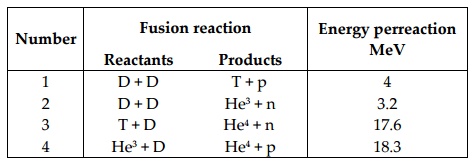
temperatures are required to trigger and sustain them. Table lists the possible
fusion reactions and the energies produced by them. n, p, D, and T are the
symbols for the neutron, proton, deuterium (H2), and tritium (H3),
respectively.
Number Fusion reaction Energy perreaction (MeV
)
Reactants Products
1 D + D T + p 4
2 D + D He3 + n 3.2
3 T + D He4 + n 17.6
4 He3
+ D He4 + p 18.3
Many
problems have to be solved before an artificially made fusion reactor becomes a
reality. The most important of these are the difficulty in generating and
maintaining high temperatures and the instabilities in the medium (plasma), the
conversion of fusion energy to electricity, and many other problems of an
operational nature.
Related Topics