Chapter: Clinical Anesthesiology: Anesthetic Equipment & Monitors : Non cardiovascular Monitoring
Evoked Potentials - Neurological System Monitors
EVOKED POTENTIALS
Indications
Indications for intraoperative
monitoring of evokedpotentials (EPs)
include surgical procedures asso-ciated with possible neurological injury:
spinal fusion with instrumentation, spine and spinal cord tumor resection,
brachial plexus repair, thoracoab-dominal aortic aneurysm repair, epilepsy
surgery, and cerebral tumor resection. Ischemia in the spi-nal cord or cerebral
cortex can be detected by EPs. EP monitoring facilitates probe localization
during stereotactic neurosurgery. Auditory EPs have also been used to assess
the effects of general anesthesia on the brain. The middle latency auditory EP
may be a more sensitive indicator than BIS in regard to anesthetic depth. The
amplitude and latency of this signal following an auditory stimulus is
influenced by anesthetics.
Contraindications
Although there are no specific
contraindications for somatosensory-evoked potentials (SEPs), this modality is
severely limited by the availability of monitoring sites, equipment, and
trained personnel. Sensitivity to anesthetic agents can also be a limit-ing
factor, particularly in children. Motor-evoked potentials (MEPs) are
contraindicated in patients with retained intracranial metal, a skull defect,
and implantable devices, as well as after seizures and any major cerebral
insult. Brain injury secondary to repetitive stimulation of the cortex and
inducement of seizures is a concern with MEPs.
Techniques & Complications
EP monitoring noninvasively assesses
neural func-tion by measuring electrophysiological responses to sensory or
motor pathway stimulation. Commonly monitored EPs are brainstem auditory evoked
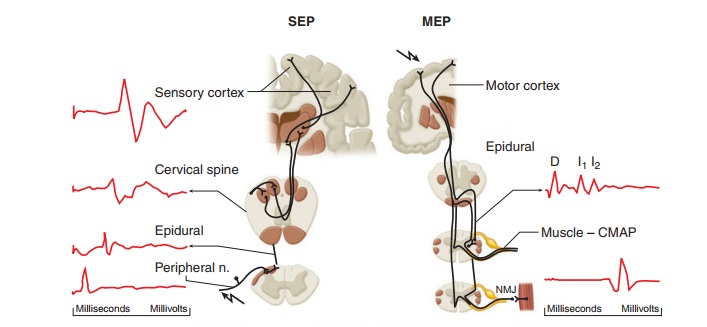
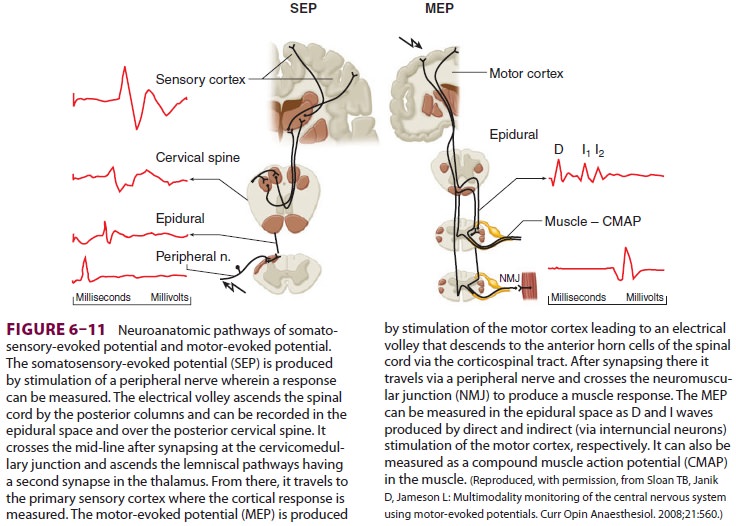
responses (BAERs), SEPs, and increasingly, MEPs (Figure 6–11).
For SEPs, a brief electrical current is
delivered to a sensory or mixed peripheral nerve by a pair of electrodes. If
the intervening pathway is intact, a nerve action potential will be transmitted
to the contralateral sensory cortex to produce an EP. This potential can be
measured by cortical surface electrodes, but is usually measured by scalp
elec-trodes. To distinguish the cortical response to a specific stimulus,
multiple responses are averaged and background noise is eliminated. EPs are
repre-sented by a plot of voltage versus time. The result-ing waveforms are
analyzed for their poststimulus latency (the time between stimulation and
potential detection) and peak amplitude. These are compared with baseline
tracings. Technical and physiological causes of a change in an EP must be
distinguished from changes due to neural damage. Complications of EP monitoring
are rare, but include skin irrita-tion and pressure ischemia at the sites of
electrode application.
Clinical Considerations
EPs are altered by many variables other
than neural damage. The effect of anesthetics is complex and not easily
summarized. In general, balanced anesthetic techniques (nitrous oxide,
neuromuscular block-ing agents, and opioids) cause minimal changes, whereas
volatile agents (halothane, sevoflurane, desflurane, and isoflurane) are best
avoided or used at a constant low dose. Early-occurring (spe-cific) EPs are
less affected by anesthetics than are late-occurring (nonspecific) responses.
Changes in BAERs may provide a measure of the depth of anes-thesia.
Physiological (eg, blood pressure, tempera-ture, and oxygen saturation) and
pharmacological factors should be kept as constant as possible.
Persistent obliteration of EPs is
predictive of postoperative neurological deficit. Although SEPs usually
identify spinal cord damage, because of their different anatomic pathways, sensory (dorsal spinal cord) EP
preservation does not guarantee normal motor
(ventral spinal cord) function (false negative). Furthermore, SEPs elicited
from pos-terior tibial nerve stimulation cannot distinguish between peripheral
and central ischemia (false posi-tive). Techniques that elicit MEPs by using
transcra-nial magnetic or electrical stimulation of the cortex allow the
detection of action potentials in the mus-cles if the neural pathway is intact.
The advantage of using MEPs as opposed to SEPs for spinal cord monitoring is
that MEPs monitor the ventral spi-nal cord, and if sensitive and specific
enough, can be used to indicate which patients might develop a postoperative
motor deficit. MEPs are more sensi-tive to spinal cord ischemia than are SEPs.
The same considerations for SEPs are applicable to MEPs in that they are
affected by volatile inhalational agents, high-dose benzodiazepines, and
moderate hypothermia (temperatures less than 32°C). MEPsrequire monitoring of
the level of neuromuscu-lar blockade. Close communication with a
neu-rophysiologist is essential prior to the start of any case where these
monitors are used to review the optimal anesthetic technique to ensure
monitoring integrity. MEPs are sensitive to volatile anesthet-ics.
Consequently, intravenous techniques are often preferred.
Related Topics