Chapter: Clinical Anesthesiology: Anesthetic Equipment & Monitors : Non cardiovascular Monitoring
Capnography
CAPNOGRAPHY
Indications & Contraindications
Determination of end-tidal CO2 (Etco2) concentra-tion to confirm adequate
ventilation is mandatory during all anesthetic procedures, but particularly so
for general anesthesia. A rapid fall of Etco2
is a sensitive indicator of air embolism, a major com-plication of sitting
craniotomies. There are no contraindications.
Techniques & Complications
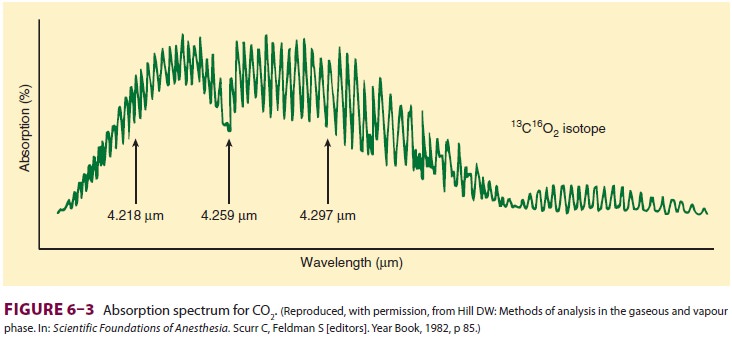
Capnography is a valuable monitor of the
pul-monary, cardiovascular, and anesthetic breathing systems. Capnographs in
common use rely on the absorption of infrared light by CO 2 (Figure 6–3). As with oximetry, absorption of
infrared light by CO2 is governed by the Beer–Lambert law.
A. Nondiverting (Flowthrough)
Nondiverting (mainstream) capnographs
mea-sure CO2 passing through an adaptor placed in
the breathing circuit (Figure 6–4). Infrared light trans-mission
through the gas is measured and CO2
con-centration is determined by the monitor. Because of problems with drift,
older flowthrough models self-zeroed during inspiration. Thus, they were
incapable
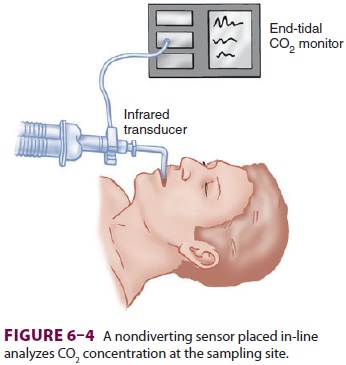
of detecting inspired
CO2, such as would occur with a breathing circuit malfunction (eg,
absorbent exhaustion, sticking unidirectional valves). The weight of the sensor
causes traction on the tracheal tube, and its generation of radiant heat can
cause skin burns. Newer designs address
these problems.
B. Diverting (Aspiration)
Diverting
(sidestream) capnographs continuously suctions gas from the breathing circuit
into a sample cell within the monitor. CO 2 concentration is
deter-mined by comparing infrared light absorption in the sample cell with a
chamber free of CO2. Continuous aspiration of anesthetic gas
essentially represents a leak in the breathing circuit that will contaminate
the operating room unless it is scavenged or returned to the breathing system.
High aspiration rates (up to 250 mL/min) and low-dead-space sampling tubing usually
increase sensitivity and decrease lag time. If tidal volumes (Vt) are small
(eg, pediatric patients), however, a high rate of aspiration may entrain fresh
gas from the circuit and dilute Etco2 measure-ment. Low aspiration
rates (less than 50 mL/min) can retard Etco2 measurement and
underestimate it during rapid ventilation. New units autocalibrate, but older
units must be zeroed to room air and against a known CO2
concentration (usually 5%). Diverting units are prone to water precipitation in
the aspiration tube and sampling cell that can causeobstruction of the sampling
line and erroneous readings. Expiratory valve malfunction is detected by the
presence of CO 2 in inspired gas. Although inspiratory valve failure
also results in rebreathing CO2, this is not as readily apparent
because part of the inspiratory volume will still be free of CO 2, caus-ing the monitor to read zero during
part of the inspi-ratory phase.
Clinical Considerations
Other
gases (eg, nitrous oxide) also absorb infrared light, leading to a
pressure-broadening effect. To minimize the error introduced by nitrous oxide,
various modifications and filters have been incorporated into monitor design.
Capnographs rap-idly and reliably indicate esophagealintubation—a common cause
of anesthetic catastro-phe—but do not reliably detect bronchial intuba-tion.
Although there may be some CO2 in the stomach from swallowing
expired air, this should be washed out within a few breaths. Sudden cessation
of CO2 during the expiratory phase may indicate a circuit
disconnection. The increased metabolic rate caused by malignant hyperthermia
causes a marked rise in Etco2.
The
gradient between Paco2 and Etco2 (nor-mally 2–5 mm Hg)
reflects alveolar dead space (alveoli that are ventilated but not perfused).
Any significant reduction in lung perfusion (eg, air embolism, decreased
cardiac output, or decreased blood pressure) increases alveolar dead space,
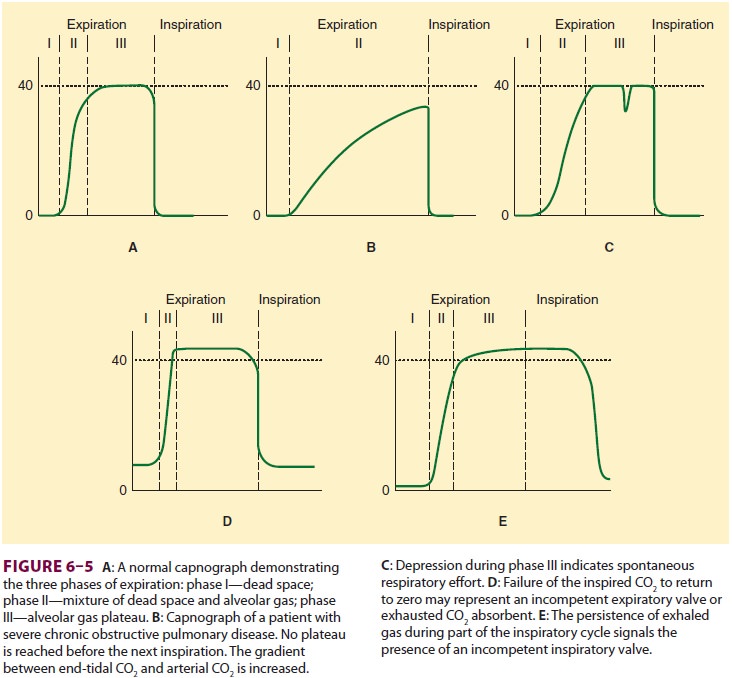
dilutes expired CO2, and lessens Etco2. True capno-graphs
(as opposed to capnometers) display a wave-form of CO 2 concentration
that allows recognition of a variety of conditions (Figure 6–5).
Related Topics