Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Molecular Biotechnology
DNA Transfer - Recombinant DNA Technology
DNA Transfer
As stated above, transfer of a recombinant DNA molecule to a cell is an
essential step in DNA technology. Some bacterial cells, like those of the
species Bacillus
subtilis which are frequently used in
industrial biotechnology, are able to take up DNA under physiological
conditions. This process is de-scribed as natural transformation. In most cases,
however, microbial cells have to be forced by an unusual regimen to take up
DNA. For example, in the case of microorganisms such non-physiological
con-ditions are created by applying a heat shock to the host cells in the
presence of high amounts of Ca2þ ions. An alternative technique used to force DNA uptake is
electroporation. For that purpose DNA and cells are brought together in a
cuvette which is then subjected to a vigorous electrical discharge. Under those
artificial conditions the cell envelope is forced to open itself, after which
DNA may enter through the “holes” that are created. The brute force in these
techniques kills a large fraction of the cells, but sufficient cells survive,
among which are several that took up DNA. The technique of electroporation is
widely applicable and frequently used.
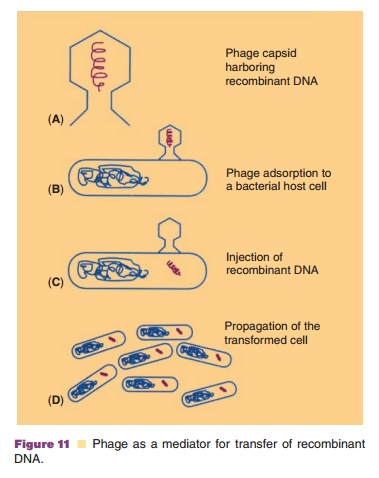
Next to direct transfer of recombinant DNA molecules as such, there is
at least for transfer to bacterial cells the possibility to package DNA in a
bacteriophage capsid and then to mimic the normal bacteriophage infection
procedure (Fig. 11). Transfer to bacterial cells can also be achieved by making
use of conjugation. Conjugation is a process where DNA transfer takes place by
cell–cell mating. For
conjugation a special class of plasmids is required, so-called
conjugative plasmids. If a cell with such a plasmid—the donor—meets a cell
without such plasmid—the recipient—they may form together cell aggregates. In
the so-called mating aggregate theplasmid from the donor has the ability to
transfer itself, as a consequence of a conjugative replication process
according to the rolling circle model, to the recipient cell. By manipulating
the conjugative plas-mids one may create donors harboring recombinant DNA
molecules which can then rather efficiently be transferred by cell–cell
contact.
If an animal virus or a plant virus is used as vector for the
recombinant DNA technology, one may exploit natural virus infection processes
to transfer DNA to an animal or a plant cell. Like the case in microorganisms,
DNA transfer to animal cells can be forced by a treatment with high amounts of
Ca2þ ions or by another chemical treatment. Next to that, it is possible to
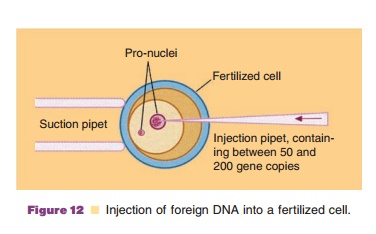
inject DNA with a syringe into the nucleus of the cell. The latter technique
(one could speak of a kind of micro-surgery) is feasible due to the relative
large dimensions of the animal cells com-pared to bacteria and is also applied
to plant cells. The technique is illustrated in Figure 12. The cell is brought
on the tip of a thin glass tube and is fixed to the tube by suction at the
other end of the tube. By means of a micromanipulator a small syringe filled
with DNA is directed to the nucleus of the fixed cell and then the DNA is
injected into the nucleus.
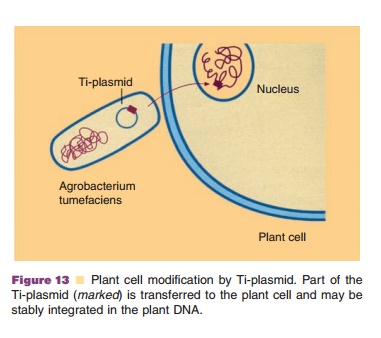
A very successful way to transfer DNA into plant systems is based on a
special type of conjuga-tion. The soil bacterium, Agrobacterium tumefaciens, harbors a conjugative plasmid called Ti (acronym for tumor inducing).
If such a bacterium infects wounded tissues of certain plants, part of the
Ti-plasmid is transferred to a plant cell in a conjugation-like process. This
transfer is followed by integration of the transferred DNA into the genome of
the plant. The infected plant cells lose normal growth control and develop a
tumor (a plant disease called crown gall). By manipulating the Ti-plasmid, such
that its tumor inducing properties are lost and foreign DNA frag-ments are
linked to it, any DNA can be transferred in a convenient way from the modified Agrobacterium donor to a plant cell. Figure 13 illustrates this remarkable process,
where, in fact, biological king-dom barriers are crossed in a natural fashion.
Since the wall of the plant cell is the main barrier for uptake of DNA,
one has exploited protoplasts of plant cells (i.e., plant cells lacking walls)
to introduce DNA. Protoplasts can take up DNA quite easily. It is feasible to
regenerate from genetically modified protoplasts intact plant cells. Finally, a
very artificial method to introduce DNA in plant tissue has been developed.
Microprojectiles covered with DNA are shot with a gun into plant cells. In
fact, many plant species that can not readily be genetically modified with any
of the methods mentioned above can be modified using this rather bizarre gun
method.
The various techniques that are used to transfer DNA are generally not
very efficient and may cause, as stated before, extensive killing of cells.
Moreover, the fate of the transferred DNA is not always predictable. For
example, in some cases the intro-duced DNA is subject to nuclease-mediated
break-down, while in animal or plant cells the introduced DNA does not always
reach the nucleus, nor is it always integrated in a proper way. All methods to
transfer DNA yield, in general, only a few cells that are vital and stable.
Therefore, selection techniques are highly desirable to find these rare cells.
Most selection techniques use a marker on the vector that codes for a selective
property. Markers which code for a resistance towards a specific antibiotic
substance are frequently used. If the cell that has to be modified is sensitive
towards that antibiotic, the few modified cells from the transfer trial can
easily be selected by bringing samples of the treated cells (either microbial
cells, plant cells or animal cells) in a medium containing the relevant
antibiotic. Only the cells that took up DNA and do maintain that DNA in their
progeny will proliferate, all other cells are killed or, at least, do not grow.
An alternative selection method uses recipient cells with specific growth
deficiencies
Related Topics