Chapter: BIOLOGY (ZOOLOGY) Standard XI first year 11th text book Assignment topics question and answer Explanation Definition
Cytological Techniques
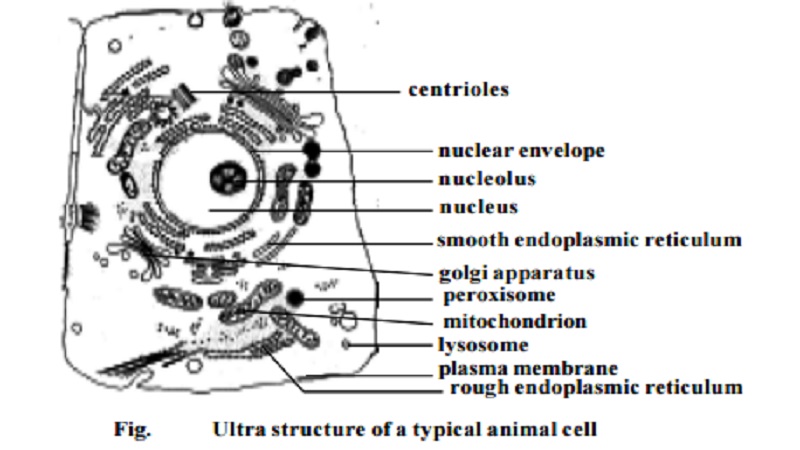
Cytological Techniques
Cells are transparent and optically homogeneous organisations. They can be observed either directly or after preservation. For direct observation, the specimen needs sufficient contrast. Direct observation is possible by using vital stains.
Vital stains :- Vital dyes or stains are taken up by living cells without killing them. They selectively stain intracellular structures without affecting cellular metabolism and function. For example, Janus green B selectively stains mitochondria, Golgi apparatus, nuclear chromatin in a dividing cell can be stained by methylene blue; Neutral Red dye or Congo Red dye can be used to stain yeast cells.
Preserved and stained tissues :- For detailed microscopic study, tissues containing cells are passed through various stages. The stages of cell prepa-ration on a glass slide involves killing, fixation, dehydration, embedding, sectioning, staining and mounting.
1). Killing and fixation :- This process causes sudden death of cells or tissues and preserves freshly killed tissues in as lifelike a condition as pos-sible. A good fixative prevents bacterial decay and autolysis. It will also make different cell components more visible and prepare the cell for staining. The commonly used fixatives are Acetic acid. Formaldehyde, Bouin's solution and Carnoy's fluid.
2). Dehydration :- In this process water vapour are removed from cells or tissues using chemical agents. It is done by using ethanol and benzene.
3). Embedding :- The tissues are infiltrated with molten paraffin wax. It hard-ens up on cooling and provides enough support to allow thin sections. Very thin sections need to be taken for electron microscopy. Hence plastics are used for embedding.
4). Sectioning :- The embedding material is cut into thin sections of needed thickness. It is done by using an instrument called microtome.
5). Staining :- The sections are immersed in dyes that stain some structures better than others. For example, cytoplasm stains pink with eosin. Nucleus stains blue with haematoxylin or red with safranin.
6). Dehydration :- Stained sections are immersed in ethanol to remove wa-ter. The tissue becomes more transparent. Dehydration is done gradually by using a series of increasing concentrations of ethanol in water. Finally the section is placed in 'absolute' alcohol.
7). Mounting :- Cleaned sections are mounted on a slide using a suitable medium like canada balsam. A cover slip is added and the medium is allowed to dry.
Related Topics