Chapter: Essential Microbiology: Viruses
Cultivating viruses
Cultivating
viruses
Whilst the growth of bacteria in the laboratory
generally demands only a supply of the relevant nutrients and appropriate
environmental conditions, maintaining viruses presents special challenges.
Think back, and you will realise why this is so; all viruses are obligate
intracellular parasites, and therefore need an appropriate host cell if they
are to replicate.
Bacteriophages, for example, are grown in culture
with their bacterial hosts. Stock cultures of phages are prepared by allowing
them to infect a broth culture of the appro-priate bacterium. Successful
propagation of phages results in a clearing of the culture’s turbidity;
centrifugation removes any remaining bacteria, leaving the phage particles in
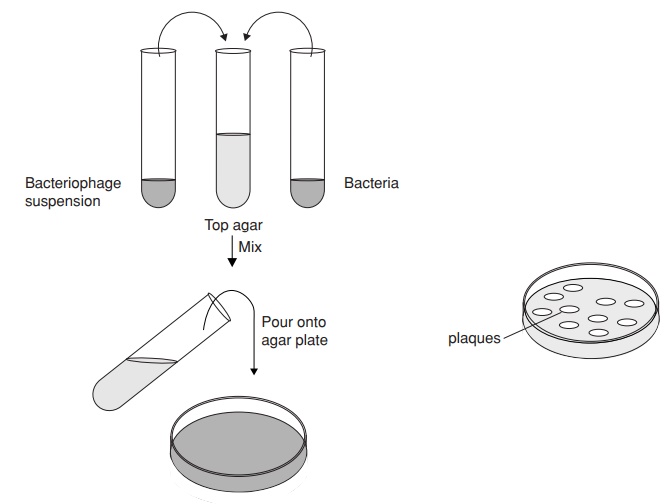
the supernatant. A quantitative measure of phages, known as the titre, can be obtained by mixing them
with a much greater number of bacteria and immobilising them in agar. Due to
their numbers, the bacteria grow as a confluent lawn. Some become infected by phage, and when new viral particles
are released following lysis of their host, they infect more host cells.
Because they are immobilised in agar, the phages are only able to infect cells
in the immediate vicinity. As more and more cells in the same area are lysed, an
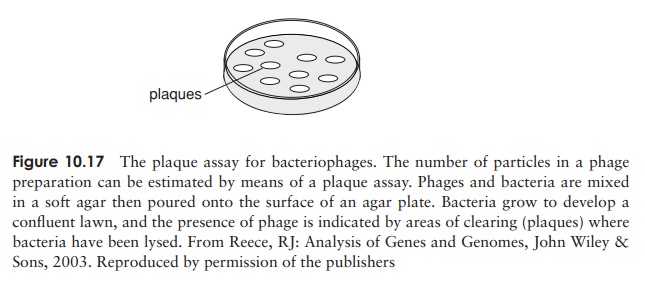
area of clearing called a plaque
appears in the lawn of bacteria (Figure 10.17). Quantification is based on the
assumption that each visible plaque arises from
Animal viruses used to be propagated in the host
animal; clearly there are limitations to this, not least when the host is
human! One of the major breakthroughs in the field of virus cultivation was
made in 1931 when it was shown by Alice Woodruff and Ernest Goodpasture that
fertilised chicken’s eggs could serve as a host for a number of animal
and human viruses, such as those that cause rabies
and influenza. It has been said that the chicken embryo did for virus culture
what agar did for the growth of bacteria. Depending on the virus in question,
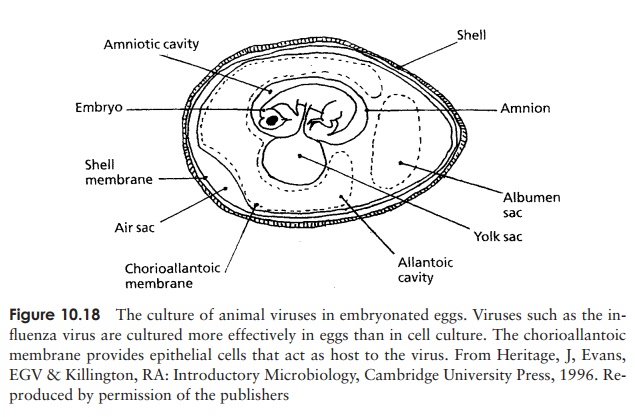
inoculation can be made into the developing embryo itself or into one of the
various membranes and cavities such as the chorioallantoic membrane or the
allantoic cavity (Figure 10.18). Viral propagation is demonstrated by death of
the embryo, or the appearance of lesions on the membranes.
In the 1950s, cell culture techniques advanced,
thanks in part to the widespread avail-ability of antibiotics, making the
control of bacterial contamination much more readily achieved. Cells are
usually grown as monolayers in tissue
culture flasks containing a suitable liquid growth medium. Treatment with the
protease trypsin dissolves the con-nective tissue matrix between the cells,
allowing them to be harvested, and used to
Plant viruses need to overcome the barrier presented
by the cellulose cell wall of the plant; in nature this is often achieved by
the piercing mouthparts of an insect vector or by entering areas of damaged
tissue. Experimentally, viruses can be introduced into an appropriate host by rubbing
the surface of a leaf with the virus together with a mild abrasive to create a
minor wound.
Related Topics