Chapter: Basic & Clinical Pharmacology : Skeletal Muscle Relaxants
Clinical Pharmacology of Neuromuscular Blocking Drugs
CLINICAL
PHARMACOLOGY OF NEUROMUSCULAR BLOCKING DRUGS
Skeletal Muscle Paralysis
Before
the introduction of neuromuscular blocking drugs, pro-found skeletal muscle
relaxation for intracavitary operations could be achieved only by producing
levels of volatile (inhaled) anesthe-sia deep enough to produce profound
depressant effects on the cardiovascular and respiratory systems. The adjunctive
use of neu-romuscular blocking drugs makes it possible to achieve adequate
muscle relaxation for all types of surgical procedures without the
cardiorespiratory depressant effects produced by deep anesthesia.
Assessment of Neuromuscular Transmission
Monitoring
the effect of muscle relaxants during surgery (and recovery following the
administration of cholinesterase inhibitors) typically involves the use of a
device that produces transdermal electrical stimulation of one of the
peripheral nerves to the hand or facial muscles and recording of the evoked
contractions (ie, twitch responses). The motor responses to different patterns
of peripheral nerve stimulation can be recorded in the operating room during
the procedure (Figure 27–7). The three most com-monly used patterns include (1)
single-twitch stimulation, (2) train-of-four (TOF) stimulation, and (3) tetanic
stimulation. Two newer modalities are also available to monitor neuromuscular
transmission: double-burst stimulation and posttetanic count.
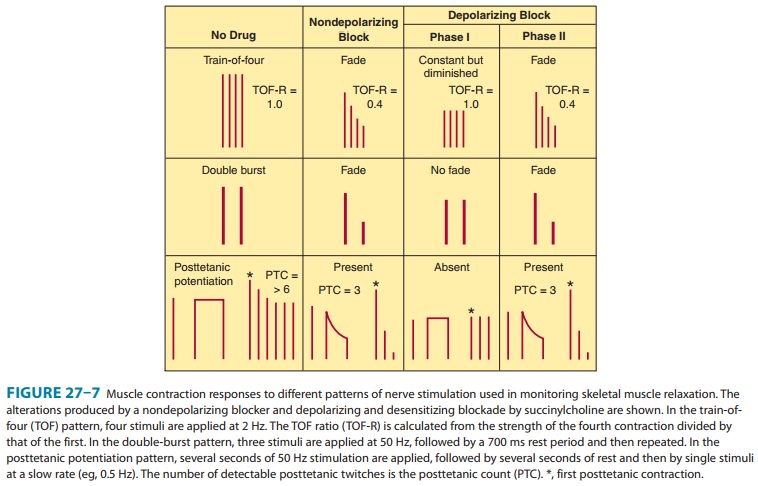
With
single-twitch stimulation, a single supramaximal electri-cal stimulus is
applied to a peripheral nerve at frequencies from 0.1 Hz to 1.0 Hz. The higher
frequency is often used during\nduction and reversal to more accurately
determine the peak (maximal) drug effect. TOF stimulation involves four
successive supramaximal stimuli given at intervals of 0.5 second (2 Hz). Each
stimulus in the TOF causes the muscle to contract, and the rela-tive magnitude
of the response of the fourth twitch compared with the first twitch is the TOF
ratio. With a depolarizing block, all four twitches are reduced in a
dose-related fashion. With a nondepolarizing block, the TOF ratio decreases
(“fades”) and is inversely proportional to the degree of blockade. During
recovery from nondepolarizing block, the amount of fade decreases and the TOF
ratio approaches 1.0. Recovery to a TOF ratio greater than 0.7 is typically
necessary for resumption of spontaneous ventila-tion. However, complete
clinical recovery from a nondepolarizing block is considered to require a TOF
greater than 0.9. Fade in the TOF response after administration of
succinylcholine signifies the development of a phase II block.
Tetanic
stimulation consists of a very rapid (30–100 Hz) deliv-ery of electrical
stimuli for several seconds. During a nondepolar-izing neuromuscular block (and
a phase II block after succinylcholine), the response is not sustained and fade
of the twitch responses is observed. Fade in response to tetanic stimula-tion
is normally considered a presynaptic event. However, the degree of fade depends
primarily on the degree of neuromuscular blockade. During a partial
nondepolarizing blockade, tetanic nerve stimulation is followed by an increase
in the posttetanic twitch response, so-called posttetanic facilitation of
neuromuscu-lar transmission. During intense neuromuscular blockade, there is no
response to either tetanic or posttetanic stimulation. As the intensity of the
block diminishes, the response to posttetanic twitch stimulation reappears. The
time to reappearance of the first response to TOF stimulation is related to the
posttetanic count and reflects the duration of profound (clinical)
neuromuscular blockade. To determine the posttetanic count, 5 seconds of 50 Hz
tetany is applied, followed by 3 seconds of rest, followed by 1 Hz pulses for
about 10 seconds (10 pulses). The counted number of muscle twitches provides an
estimation of the depth of blockade. For instance, a posttetanic count of 2
suggests no twitch response (by TOF) for about 20–30 minutes, and a posttetanic
count of 5 correlates to a no-twitch response (by TOF) of about 10–15 minutes
(Figure 27–7, bottom panel).
The
double-burst stimulation pattern is a newer mode of elec-trical nerve
stimulation developed with the goal of allowing for manual detection of
residual neuromuscular blockade when it is not possible to record the responses
to single-twitch, TOF, or tetanic stimulation. In this pattern, three nerve
stimuli are deliv-ered at 50 Hz followed by a 700 ms rest period and then by
two or three additional stimuli at 50 Hz. It is easier to detect fade in the
responses to double-burst stimulation than to TOF stimula-tion. The absence of
fade in response to double-burst stimulation implies that clinically
significant residual neuromuscular blockade does not exist.
The
standard approach used for monitoring the clinical effects of muscle relaxants
during surgery is to use a peripheral nerve stimulating device to elicit motor
responses, which are visually observed by the anesthesiologist. A more
quantitative approach to neuromuscular monitoring involves the use of
acceleromyography or force-transduction for measuring the evoked response (ie,
movement) of the thumb to TOF stimulation over the ulnar nerve at the wrist.
A. Nondepolarizing Relaxant Drugs
During
anesthesia, administration of tubocurarine, 0.1–0.4 mg/ kg IV, initially causes
motor weakness, followed by the skeletal muscles becoming flaccid and
inexcitable to electrical stimula-tion (Figure 27–9). In general, larger
muscles (eg, abdominal, trunk, paraspinous, diaphragm) are more resistant to
neuromus-cular blockade and recover more rapidly than smaller muscles (eg,
facial, foot, hand). The diaphragm is usually the last muscleto be paralyzed.
Assuming that ventilation is adequately main-tained, no adverse effects occur.
When administration of muscle relaxants is discontinued, recovery of muscles
usually occurs in reverse order, with the diaphragm regaining function first.
The pharmacologic effect of tubocurarine, 0.3 mg/kg IV, usually lasts 45–60
minutes. However, subtle evidence of residual muscle paralysis detected using a
neuromuscular monitor may last for another hour, increasing the likelihood of
adverse outcomes, eg, aspiration and decreased hypoxic drive. Potency and
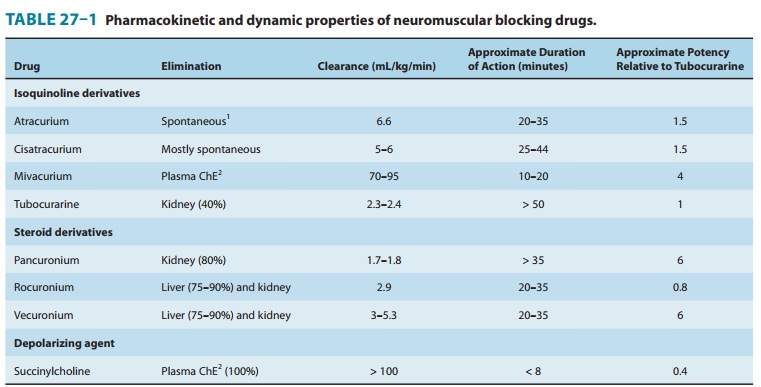
duration of action of the other nondepolarizing drugs are shown in Table 27–1.
In addition to the duration of action, the most important property
distinguishing the nondepolarizing relaxants is the time to onset of the
blocking effect, which determines how rapidly the patient’s trachea can be
intubated. Of the currently available nondepolarizing drugs, rocuronium has the
most rapid onset time (60–120 seconds).
B. Depolarizing Relaxant Drugs
Following
the administration of succinylcholine, 0.75–1.5 mg/kg IV, transient muscle
fasciculations occur over the chest and abdo-men within 30 seconds, although
general anesthesia and the prior administration of a small dose of a
nondepolarizing muscle relax-ant tends to attenuate them. As paralysis develops
rapidly (< 90 seconds), the arm, neck, and leg muscles are initially relaxed
fol-lowed by the respiratory muscles. As a result of succinylcholine’s rapid
hydrolysis by cholinesterase in the plasma (and liver), the duration of
neuromuscular block typically lasts less than 10 min-utes (Table 27–1).
Cardiovascular Effects
Vecuronium,
cisatracurium, and rocuronium have minimal, if any, cardiovascular effects. The
other nondepolarizing muscle relaxants (ie, pancuronium, atracurium,
mivacurium) produce cardiovascular effects that are mediated by either
autonomic or
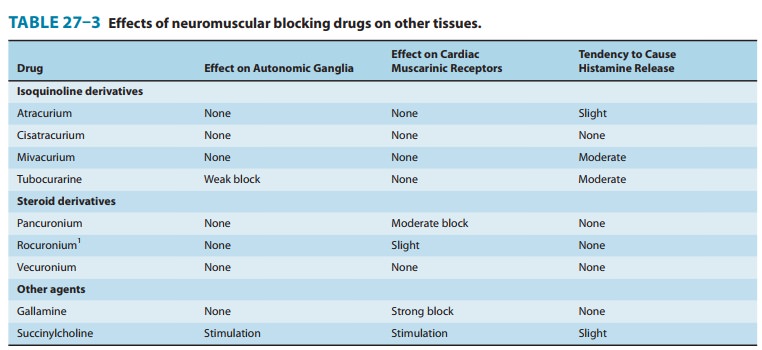
histamine
receptors (Table 27–3). Tubocurarine and, to a lesser extent, metocurine,
mivacurium, and atracurium can produce hypotension as a result of systemic
histamine release, and with larger doses, ganglionic blockade may occur with
tubocurarine and metocurine. Premedication with an antihistaminic compound
attenuates tubocurarine- and mivacurium-induced hypotension. Pancuronium causes
a moderate increase in heart rate and a smaller increase in cardiac output,
with little or no change in sys-temic vascular resistance. Although
pancuronium-induced tachy-cardia is primarily due to a vagolytic action,
release of norepinephrine from adrenergic nerve endings and blockade of neuronal
uptake of norepinephrine may be secondary mecha-nisms. Bronchospasm may be
produced by neuromuscular blockers that release histamine (eg, mivacurium), but
insertion of a endo-tracheal tube is the most common reason for bronchospasm
after induction of general anesthesia.
Succinylcholine
can cause cardiac arrhythmias when admin-istered during halothane anesthesia.
The drug stimulates auto-nomic cholinoceptors, including the nicotinic
receptors at both sympathetic and parasympathetic ganglia and muscarinic recep-tors
in the heart (eg, sinus node). The negative inotropic and chronotropic
responses to succinylcholine can be attenuated by administration of an
anticholinergic drug (eg, glycopyrrolate, atropine). With large doses of
succinylcholine, positive inotro-pic and chronotropic effects may be observed.
On the other hand, bradycardia has been repeatedly observed when a second dose
of succinylcholine is given less than 5 minutes after the initial dose. This
transient bradycardia can be prevented by thiopental, atropine,
ganglionic-blocking drugs, and by pre-treating with a small dose of a
nondepolarizing muscle relaxant (eg, pancuronium). Direct myocardial effects,
increased musca-rinic stimulation, and ganglionic stimulation contribute to
this bradycardic response.
Other Adverse Effects of Depolarizing Blockade
A. Hyperkalemia
Patients
with burns, nerve damage or neuromuscular disease, closed head injury, and
other trauma can respond to succinylcho-line by releasing potassium into the
blood, which, on rare occa-sions, results in cardiac arrest.
B. Increased Intraocular Pressure
Administration
of succinylcholine may be associated with the rapid onset of an increase in
intraocular pressure (< 60 seconds), peaking at 2–4 minutes, and declining
after 5 minutes. The mechanism may involve tonic contraction of myofibrils or
tran-sient dilation of ocular choroidal blood vessels. Despite the increase in
intraocular pressure, the use of succinylcholine for ophthalmologic operations
is not contraindicated unless the ante-rior chamber is open (“open globe”) due
to trauma.
C. Increased Intragastric Pressure
In
heavily muscled patients, the fasciculations associated with suc-cinylcholine
may cause an increase in intragastric pressure ranging from 5 to 40 cm H2O,
increasing the risk for regurgitation and aspiration of gastric contents. This
complication is more likely to occur in patients with delayed gastric emptying
(eg, those with diabetes), traumatic injury (eg, an emergency case), esophageal
dysfunction, and morbid obesity.
D. Muscle Pain
Myalgias
are a common postoperative complaint of heavily mus-cled patients and those who
receive large doses (> 1.5 mg/kg) of succinylcholine. The true incidence of
myalgias related to muscle fasciculations is difficult to establish because of
confounding
However, the incidence of myal-gias has been reported to vary from
less than 1% to 20%. It occurs more frequently in ambulatory than in bedridden
patients. The pain is thought to be secondary to the unsynchronized
contrac-tions of adjacent muscle fibers just before the onset of paralysis.
However, there is controversy over whether the incidence of muscle pain
following succinylcholine is actually higher than with nondepolarizing muscle
relaxants when other potentially con-founding factors are taken into
consideration.
Interactions with Other Drugs
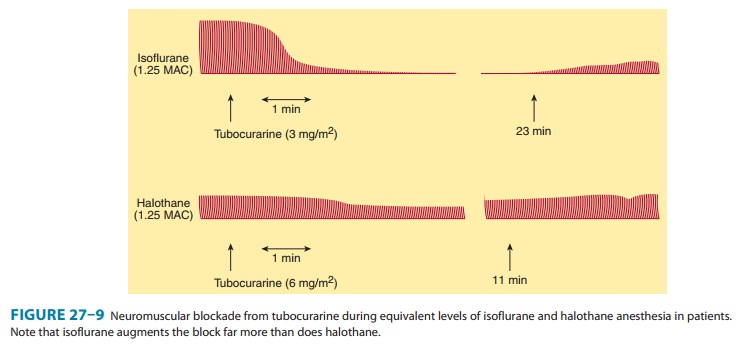
A. Anesthetics
Inhaled
(volatile) anesthetics potentiate the neuromuscular block-ade produced by
nondepolarizing muscle relaxants in a dose-de-pendent fashion. Of the general
anesthetics that have been studied, inhaled anesthetics augment the effects of
muscle relax-ants in the following order: isoflurane (most); sevoflurane,
desflu-rane, enflurane, and halothane; and nitrous oxide (least) (Figure 27–9).
The most important factors involved in this interaction are the following: (1)
nervous system depression at sites proximal to the neuromuscular junction (ie,
central nervous system); (2) increased muscle blood flow (ie, due to peripheral
vasodilation produced by volatile anesthetics), which allows a larger fraction
of the injected muscle relaxant to reach the neuro-muscular junction; and (3)
decreased sensitivity of the postjunc-tional membrane to depolarization.
A
rare interaction of succinylcholine with volatile anesthetics results in malignant hyperthermia, a condition
caused by abnor-mal release of calcium from stores in skeletal muscle.
B. Antibiotics
Numerous
reports have described enhancement of neuromuscu-lar blockade by antibiotics
(eg, aminoglycosides). Many of the antibiotics have been shown to cause a
depression of evoked release of acetylcholine similar to that caused by
administering magnesium. The mechanism of this prejunctional effect appears to
be blockade of specific P-type calcium channels in the motor nerve terminal.
C. Local Anesthetics and Antiarrhythmic Drugs
In
small doses, local anesthetics can depress posttetanic potentia-tion via a
prejunctional neural effect. In large doses, local anesthet-ics can block
neuromuscular transmission. With higher doses, local anesthetics block
acetylcholine-induced muscle contractions as a result of blockade of the
nicotinic receptor ion channels. Experimentally, similar effects can be
demonstrated with sodium channel-blocking antiarrhythmic drugs such as
quinidine. However, at the doses used for cardiac arrhythmias, this
interac-tion is of little or no clinical significance. Higher concentrations of
bupivacaine (0.75%) have been associated with cardiac arrhyth-mias independent
of the muscle relaxant used.
D. Other Neuromuscular Blocking Drugs
The
end plate-depolarizing effect of succinylcholine can be antag-onized by
administering a small dose of a nondepolarizing blocker. To prevent the
fasciculations associated with succinylcholine administration, a small
nonparalyzing dose of a nondepolarizing drug can be given before
succinylcholine (eg, d-tubocurarine,
2 mg IV, or pancuronium, 0.5 mg IV). Although this dose usually reduces
fasciculations and postoperative myalgias, it can increase the amount of
succinylcholine required for relaxation by 50–90% and can produce a feeling of
weakness in awake patients. Therefore, “pre-curarization” before
succinylcholine is no longer widely practiced.
Effects of Diseases & Aging on the Neuromuscular Response
Several
diseases can diminish or augment the neuromuscular blockade produced by
nondepolarizing muscle relaxants. Myasthenia gravis enhances the neuromuscular
blockade pro-duced by these drugs. Advanced age is associated with a prolonged
duration of action from nondepolarizing relaxants as a result of decreased
clearance of the drugs by the liver and kidneys. As a result, the dosage of
neuromuscular blocking drugs should be reduced in older patients (> 70
years).
Conversely,
patients with severe burns and those with upper motor neuron disease are
resistant to nondepolarizing muscle relaxants. This desensitization is probably
caused by proliferation of extrajunctional receptors, which results in an
increased dose requirement for the nondepolarizing relaxant to block a
sufficient number of receptors.
Reversal of Nondepolarizing Neuromuscular Blockade
The
cholinesterase inhibitors effectively antagonize the neuromus-cular blockade
caused by nondepolarizing drugs. Neostigmine
and pyridostigmine antagonize
nondepolarizing neuromuscular block-ade by increasing the availability of
acetylcholine at the motor end plate, mainly by inhibition of
acetylcholinesterase. To a lesser extent, these cholinesterase inhibitors also
increase the release of this transmitter from the motor nerve terminal. In
contrast, edrophonium antagonizes
neuromuscular blockade purely byinhibiting acetylcholinesterase activity.
Edrophonium has a more rapid onset of action but may be less effective than
neostigmine in reversing the effects of nondepolarizing blockers in the
presence of a profound degree of neuromuscular blockade. These differences are
important in determining recovery from residual
block, the neuromuscular blockade remaining after completion of surgery and
movement of the patient to the recovery room. Unsuspected residual block may
result in hypoventilation, leading to hypoxia and even apnea, especially if
patients have received central depres-sant medications in the early recovery
period.
Since
mivacurium is metabolized by plasma cholinesterase, its interaction with the
anticholinesterase reversal drugs is less pre-dictable. On the one hand, the
neuromuscular blockade is antagonized because of increased acetylcholine
concentrations in the synapse. On the other hand, mivacurium concentration may
be higher because of decreased plasma cholinesterase breakdown of the muscle
relaxant itself.
Sugammadex
is a novel reversal agent approved in Europe but not yet approved for use in
the USA. It is a modified γ-cyclodextran that binds tightly to rocuronium
in a 1:1 ratio. By binding to plasma rocuronium, sugammadex decreases the free
plasma rocuronium concentration and establishes a concentration gradi-ent for
rocuronium to diffuse away from the neuromuscular junc-tion back into the
circulation, where it is quickly bound by free sugammadex.
The
optimum dose of sugammadex required to achieve ade-quate reversal of the
neuromuscular blocking agent has yet to be definitively established. Clinical
trials studying safety and efficacy have used doses ranging from 0.5 to 16
mg/kg. From 2 mg/kg upward, sugammadex dose-dependently reverses rocuronium
with increasing speed and efficacy. These trials reported no difference in
prevalence of untoward effects among sugammadex, placebo, and neostigmine. The
data need to be confirmed in larger trials, especially the drug’s potential to
elicit allergic or hypersensitivity reactions.
The
sugammadex-rocuronium complex is typically excreted unchanged in the urine
within 24 hours in patients with normal renal function. In patients with renal
insufficiency, complete uri-nary elimination may take much longer. However, due
to the strong complex formation with rocuronium, no signs of recur-rence of
neuromuscular blockade have been noted up to 48 hours after use in such
patients.
Further
large-scale studies will be needed to evaluate the effi-cacy, safety, and
clearance of sugammadex in patient populations with various levels of renal
failure.
Uses of Neuromuscular Blocking Drugs
A. Surgical Relaxation
One
of the most important applications of the neuromuscular blockers is in
facilitating intracavitary surgery, especially in intra-abdominal and
intrathoracic procedures.
B. Endotracheal Intubation
By
relaxing the pharyngeal and laryngeal muscles, neuromuscular blocking drugs
facilitate laryngoscopy and placement of the endo-tracheal tube. Placement of a
endotracheal tube ensures an ade-quate airway and minimizes the risk of
pulmonary aspiration during general anesthesia.
C. Control of Ventilation
In
critically ill patients who have ventilatory failure from various causes (eg,
severe bronchospasm, pneumonia, chronic obstructive airway disease), it may be
necessary to control ventilation to pro-vide adequate gas exchange and to
prevent atelectasis. In the ICU, neuromuscular blocking drugs are frequently
administered to reduce chest wall resistance (ie, improve thoracic compliance)
and ineffective spontaneous ventilation in intubated patients.
D. Treatment of Convulsions
Neuromuscular
blocking drugs (ie, succinylcholine) are occasion-ally used to attenuate the
peripheral (motor) manifestations of convulsions associated with status
epilepticus or local anesthetic toxicity. Although this approach is effective
in eliminating the muscular manifestations of the seizures, it has no effect on
the central processes because neuromuscular blocking drugs do not cross the
blood-brain barrier.
Related Topics