Chapter: Medical Surgical Nursing: Management of Patients With Complications From Heart Disease
Chronic Heart Failure
CHRONIC
HEART FAILURE
As
with coronary artery disease, the incidence of HF increases with age. However,
the rate of coronary artery disease is decreasing and just the opposite is true
for HF. Nearly 5 million people in the United States have HF, with more than
one-half million new cases diagnosed each year (American Heart Association,
2001). The prevalence rate of HF among non-Hispanic whites 20 years of age or
older is 2.3% for men and 1.5% for women; for non-Hispanic blacks, the rates
are 3.5% and 3.1%, respectively (American Heart Association, 2001). HF is the
most common reason for hospital-ization of people older than age 65 and the
second most common reason for visits to a physician’s office. The rate of
readmission to the hospital remains staggeringly high. The rise in the
incidence of HF reflects the increased number of elderly and improvements in
treatment of HF resulting in increased survival rates. However, the economic
burden caused by HF is estimated to be more than 23 billion dollars in direct
and indirect costs and is expected to in-crease (American Heart Association,
2001). Many hospitalizations could be prevented by improved and appropriate
outpatient care. Prevention and early intervention to arrest the progression of
HF are major health initiatives in the United States.
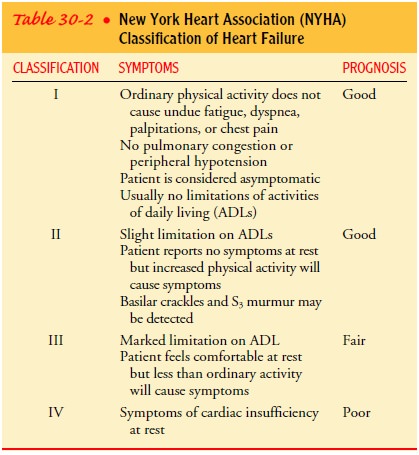
Medical management is based on the type, severity, and cause of HF. There are two types of HF, which are identified by assessment of left ventricular functioning: an alteration in ventricular filling (diastolic heart failure) and an alteration in ventricular contraction (systolic heart failure).
An assessment
of the ejection fraction(EF) is
performed to assist in determining the type of HF. EF is thepercentage of the
end-diastolic blood volume in the ventricle minus the end-systolic blood volume
in the ventricle divided by the end-diastolic blood volume in the ventricle—an
indication of the amount of blood that was ejected and the contractile ability
of the ventricle. The EF is normal in diastolic HF, whereas the EF is less than
40% in systolic HF. The severity of HF is frequently clas-sified according to
the patient’s symptoms. The New York Heart Association classification is
described in Table 30-2, and the causes are explained in subsequent sections.
Pathophysiology
HF
results from a variety of cardiovascular diseases but leads to some common
heart abnormalities that result in decreased contraction (systole), decreased
filling (diastole), or both. Signifi-cant myocardial dysfunction most often
occurs before the patient experiences signs and symptoms of HF.
Systolic HF decreases the amount of blood ejected from the ventricle, which stimulates the sympathetic nervous system to re-lease epinephrine and norepinephrine. The purpose of this initial response is to support the failing myocardium, but the continued response causes loss of beta1-adrenergic receptor sites (down-regulation) and further damage to the heart muscle cells.
The sympathetic stimulation and the decrease in renal perfusion by the failing heart cause the release of renin by the kidney. Renin promotes the formation of angiotensin I, a benign, inactive sub-stance. Angiotensin-converting enzyme (ACE) in the lumen of blood vessels converts angiotensin I to angiotensin II, a vaso-constrictor that also causes the release of aldosterone. Aldo-sterone promotes sodium and fluid retention and stimulates the thirst center. Aldosterone causes additional detrimental effects to the myocardium and exacerbates myocardial fibrosis (Pitt et al., 1999; Weber, 2001). Angiotensin, aldosterone, and other neurohormones (eg, atrial natriuretic factor, endothelin, and prostacyclin) lead to an increase in preload and afterload, which increases stress on the ventricular wall, causing an increase in the workload of the heart.
As the
heart’s workload increases, contractility of the myofi-brils decreases.
Decreased contractility results in an increase in end-diastolic blood volume in
the ventricle, stretching the myo-fibers and increasing the size of the
ventricle (ventricular dilation). The increased size of the ventricle further
increases the stress on the ventricular wall, adding to the workload of the
heart. One way the heart compensates for the increased workload is to in-crease
the thickness of the heart muscle (ventricular hypertrophy). However, the
hypertrophy is not accompanied by an adequate in-crease in capillary blood
supply, resulting in myocardial ischemia. The sympathetic-induced coronary
artery vasoconstriction, in-creased ventricular wall stress, and decreased
mitochondrial en-ergy production also lead to myocardial ischemia. Eventually,
the myocardial ischemia causes myofibril death, even in patients with-out
coronary artery disease. The compensatory mechanisms of HF have been called the
“vicious cycle of HF” because the heart does not pump sufficient blood to the
body, which causes the body to stimulate the heart to work harder; the heart is
unable to respond and failure becomes worse.
Diastolic
HF develops because of continued increased work-load on the heart, which
responds by increasing the number and size of myocardial cells (ie, ventricular
hypertrophy and altered myocellular functioning). These responses cause
resistance to ventricular filling, which increases ventricular filling
pressures de-spite a normal or reduced blood volume. Less blood in the
ven-tricles causes decreased CO. The low CO and high ventricular filling
pressures cause the same neurohormonal responses as described for systolic HF.
Etiology
Myocardial
dysfunction is most often caused by coronary artery disease, cardiomyopathy,
hypertension, or valvular disorders. Ath-erosclerosis of the coronary arteries
is the primary cause of HF. Coronary artery disease is found in more than 60%
of the patients with HF (Braunwald et al., 2001). Ischemia causes myocardial
dysfunction because of resulting hypoxia and acidosis from the ac-cumulation of
lactic acid. Myocardial infarction causes focal heart muscle necrosis, the
death of heart muscle cells, and a loss of con-tractility; the extent of the
infarction correlates with the severity of HF. Revascularization of the
coronary artery by a percutaneous coronary intervention or by coronary artery
bypass surgery may correct the underlying cause so that HF is resolved.
Cardiomyopathy
is a disease of the myocardium. There are three types: dilated, hypertrophic,
and restrictive. Dilated cardiomyopathy, the most common type of
cardio-myopathy, causes diffuse cellular necrosis, leading to decreased
contractility (systolic failure). Dilated cardiomyopathy can be id-iopathic
(unknown cause), or it can result from an inflammatory process, such as
myocarditis, from pregnancy, or from a cytotoxic agent, such as alcohol or
adriamycin. Hypertrophic cardiomy-opathy and restrictive cardiomyopathy lead to
decreased disten-sibility and ventricular filling (diastolic failure). Usually,
HF due to cardiomyopathy becomes chronic. However, cardiomyopathy and HF may
resolve after the end of pregnancy or with the ces-sation of alcohol ingestion.
Systemic
or pulmonary hypertension increases afterload (resis-tance to ejection), which
increases the workload of the heart and leads to hypertrophy of myocardial
muscle fibers; this can be con-sidered a compensatory mechanism because it
increases contrac-tility. However, the hypertrophy may impair the heart’s
ability to fill properly during diastole.
Valvular
heart disease is also a cause of HF. The valves ensure that blood flows in one
direction. With valvular dysfunction, blood has increasing difficulty moving
forward, increasing pres-sure within the heart and increasing cardiac workload,
leading to diastolic HF.
Several
systemic conditions contribute to the development and severity of HF, including
increased metabolic rate (eg, fever, thyrotoxicosis), iron overload (eg, from
hemochromatosis), hypoxia, and anemia (serum hematocrit less than 25%). All of
these conditions require an increase in CO to satisfy the sys-temic oxygen
demand. Hypoxia or anemia also may decrease the supply of oxygen to the
myocardium. Cardiac dysrhythmias may cause HF, or they may be a result of HF;
either way, the altered electrical stimulation impairs the myocardial
contraction and de-creases the overall efficiency of myocardial function. Other
factors, such as acidosis (respiratory or metabolic), electrolyte
abnor-malities, and antiarrhythmic medications, can worsen the myo-cardial
dysfunction.
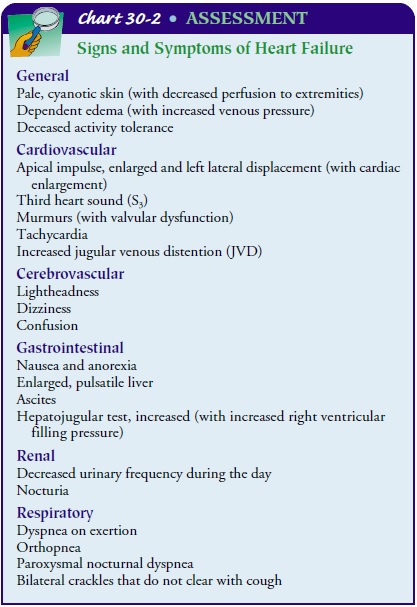
Clinical Manifestations
The
clinical manifestations produced by the different types of HF (systolic,
diastolic, or both) are similar (Chart 30-2) and there-fore do not assist in
differentiating the types of HF. The signs and symptoms of HF are most often
described in terms of the effect on the ventricles. Left-sided heart failure (left ventricular fail-ure) causes
different manifestations than
right-sided heart fail-ure (right ventricular failure). Chronic HF produces
signs andsymptoms of failure of both ventricles. Although dysrhythmias
(especially tachycardias, ventricular ectopic beats, or atrioven-tricular [AV]
and ventricular conduction defects) are common in HF, they may also be a result
of treatments used in HF (eg, side effect of digitalis).
LEFT-SIDED HEART FAILURE
Pulmonary congestion occurs when the left ventricle cannot pump the blood out of the ventricle to the body. The increased left ven-tricular end-diastolic blood volume increases the left ventricular end-diastolic pressure, which decreases blood flow from the left atrium into the left ventricle during diastole. The blood volume and pressure in the left atrium increases, which decreases blood flow from the pulmonary vessels. Pulmonary venous blood vol-ume and pressure rise, forcing fluid from the pulmonary capillaryies into the pulmonary tissues and alveoli, which impairs gas ex-change.
These effects
of left ventricular failure have been referred to as backward failure. The clinical manifestations of pulmonary venous
congestion include dyspnea, cough, pulmonary crackles, and lower-than-normal
oxygen saturation levels. An extra heart sound, S3, may be detected on auscultation.
Dyspnea,
or shortness of breath, may be precipitated by mini-mal to moderate activity (dyspnea on exertion [DOE]); dyspnea
also can occur at rest. The patient may report orthopnea, difficulty in breathing when lying flat. Patients with
orthopnea usually pre-fer not to lie flat. They may need pillows to prop
themselves up in bed, or they may sit in a chair and even sleep sitting up.
Some pa-tients have sudden attacks of orthopnea at night, a condition known as paroxysmal nocturnal dyspnea (PND).
Fluid that ac-cumulated in the dependent extremities during the day begins to
be reabsorbed into the circulating blood volume when the person lies down.
Because the impaired left ventricle cannot eject the in-creased circulating
blood volume, the pressure in the pulmonary circulation increases, causing
further shifting of fluid into the alve-oli. The fluid filled alveoli cannot
exchange oxygen and carbon dioxide. Without sufficient oxygen, the patient
experiences dys-pnea and has difficulty getting an adequate amount of sleep.
The
cough associated with left ventricular failure is initially dry and
nonproductive. Most often, patients complain of a dry hacking cough that may be
mislabeled as asthma or chronic ob-structive pulmonary disease (COPD). The
cough may become moist. Large quantities of frothy sputum, which is sometimes
pink (blood tinged), may be produced, usually indicating severe pulmonary
congestion (pulmonary edema).
Adventitious
breath sounds may be heard in various lobes of the lungs. Usually, bi-basilar
crackles that do not clear with coughing are detected in the early phase of
left ventricular failure. As the fail-ure worsens and pulmonary congestion
increases, crackles may be auscultated throughout all lung fields. At this
point, a decrease in oxygen saturation may occur.
In
addition to increased pulmonary pressures that cause de-creased oxygenation,
the amount of blood ejected from the left ventricle may decrease, sometimes
called forward failure. The dominant
feature in HF is inadequate tissue perfusion. The di-minished CO has widespread
manifestations because not enough blood reaches all the tissues and organs (low
perfusion) to pro-vide the necessary oxygen. The decrease in SV can also lead
to stimulation of the sympathetic nervous system, which further im-pedes
perfusion to many organs.
Blood
flow to the kidneys decreases, causing decreased perfu-sion and reduced urine
output (oliguria). Renal perfusion
pres-sure falls, which results in the release of renin from the kidney.Release
of renin leads to aldosterone secretion. Aldosterone se-cretion causes sodium
and fluid retention, which further increases intravascular volume. However,
when the patient is sleeping, the cardiac workload is decreased, improving
renal perfusion, which then leads to frequent urination at night (nocturia).
Decreased
CO causes other symptoms. Decreased gastro-intestinal perfusion causes altered
digestion. Decreased brain per-fusion causes dizziness, lightheadedness,
confusion, restlessness, and anxiety due to decreased oxygenation and blood
flow. As anx-iety increases, so does dyspnea, enhancing anxiety and creating a
vicious cycle. Stimulation of the sympathetic system also causes the peripheral
blood vessels to constrict, so the skin appears pale or ashen and feels cool
and clammy.
The
decrease in the ejected ventricular volume causes the sympathetic nervous
system to increase the heart rate (tachy-cardia), often causing the patient to
complain of palpitations. The pulses become weak and thready. Without adequate
CO, the body cannot respond to increased energy demands, and the patient is
easily fatigued and has decreased activity tolerance. Fatigue also results from
the increased energy expended in breath-ing and the insomnia that results from
respiratory distress, cough-ing, and nocturia.
RIGHT-SIDED HEART FAILURE
When
the right ventricle fails, congestion of the viscera and the peripheral tissues
predominates. This occurs because the right side of the heart cannot eject
blood and cannot accommodate all the blood that normally returns to it from the
venous circu-lation. The increase in venous pressure leads to jugular vein
dis-tention ( JVD).
The
clinical manifestations that ensue include edema of the lower extremities
(dependent edema), hepatomegaly (enlarge-ment of the liver), distended jugular
veins, ascites (accumulation of fluid in the peritoneal cavity), weakness,
anorexia and nausea, and paradoxically, weight gain due to retention of fluid.
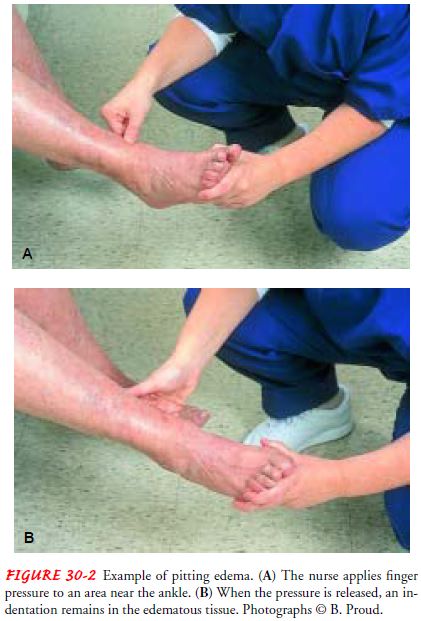
Edema
usually affects the feet and ankles, worsening when the patient stands or
dangles the legs. The swelling decreases when the patient elevates the legs.
The edema can gradually progress up the legs and thighs and eventually into the
external genitalia and lower trunk. Edema in the abdomen, as evidenced by
increased abdominal girth, may be the only edema present. Sacral edema is not
uncommon for patients who are on bed rest, because the sacral area is
dependent. Pitting edema, in whichindentations in the skin remain after even
slight compression with the fingertips (Fig. 30-2), is obvious only after
retention of at least 4.5 kg (10 lb) of fluid (4.5 liters).
Hepatomegaly
and tenderness in the right upper quadrant of the abdomen result from venous
engorgement of the liver. The in-creased pressure may interfere with the
liver’s ability to perform (secondary liver dysfunction). As hepatic
dysfunction progresses, pressure within the portal vessels may rise enough to
force fluid into the abdominal cavity, a condition known as ascites. This
col-lection of fluid in the abdominal cavity may increase pressure on the
stomach and intestines and cause gastrointestinal distress. He-patomegaly may
also increase pressure on the diaphragm, causing respiratory distress.
Anorexia
(loss of appetite) and nausea or abdominal pain re-sults from the venous
engorgement and venous stasis within the abdominal organs. The weakness that
accompanies right-sided HF results from reduced CO, impaired circulation, and
in-adequate removal of catabolic waste products from the tissues.
Assessment and Diagnostic Findings
HF may go undetected until the patient presents with signs and symptoms of pulmonary and peripheral edema (congestion), which can lead the physician to make a preliminary diagnosis of CHF. However, the physical signs that suggest HF may also occur with other diseases, such as renal failure, liver failure, oncologic conditions, and COPD. If further assessment and evaluation are not completed, these patients may be treated for HF inappropri-ately.
The term congestive heart failure (CHF) means the
patient has a fluid overload condition (congestion) that may or may not be
caused by HF. CHF is caused by HF when ventricular dys-function (systolic,
diastolic, or both) has been identified. Assess-ment of ventricular function is
an essential part of the initial diagnostic workup.
An
echocardiogram is usually performed to confirm the diag-nosis of HF, assist in
the identification of the underlying cause, and determine the patient’s
ejection fraction, which assists in identifi-cation of the type and severity of
HF. This information may also be obtained noninvasively by radionuclide
ventriculography or in-vasively by ventriculogram as part of a cardiac
catheterization pro-cedure. A chest x-ray and an electrocardiogram (ECG) are
obtained to assist in the diagnosis and to determine the underlying cause of
HF. Laboratory studies usually completed in the initial workup in-clude serum electrolytes,
blood urea nitrogen (BUN), creatinine, B-type natriuretic peptide (BNP),
thyroid-stimulating hormone (TSH), a complete blood cell count (CBC), and
routine urinaly-sis. The results of these laboratory studies assist in
determining the underlying cause and in establishing a baseline from which to
mea-sure effects of treatment. Exercise testing or cardiac catheterization may
be performed to determine whether coronary artery disease and cardiac ischemia
are causing the HF.
Ventricular
function should be determined before discharge from a hospital of patients with
acute myocardial infarction (MI) who are at risk for the development of HF.
Patients who are at low risk for HF are those who meet all of the following
criteria: no pre-vious myocardial infarction, inferior myocardial infarction,
small (less than two to four times normal) increase in cardiac enzymes, no Q
waves on the ECG, and an uncomplicated clinical course (AHCPR, 1994).
Evaluation of ventricular function may also be performed for patients whose
initial assessment of HF suggested noncardiac causes but who failed to respond
to treatment.
Medical Management
A
critical step in the management of HF is early identification and documentation
of the type of HF. Medical management, especially the pharmacologic therapy,
varies with the type of HF. The basic objectives in treating patients with HF
are the following:
· Eliminate or reduce any
etiologic contributory factors, es-pecially those that may be reversible, such
as atrial fibrilla-tion or excessive alcohol ingestion.
· Reduce the workload on
the heart by reducing afterload and preload.
Managing
the patient with HF includes providing general counseling and education about
sodium restriction, monitoring daily weights and other signs of fluid retention,
encouraging reg-ular exercise, and recommending avoidance of excessive fluid
in-take, alcohol, and smoking. Medications are prescribed based on the
patient’s type and severity of HF. Oxygen therapy is based on the degree of
pulmonary congestion and resulting hypoxia. Some patients may need supplemental
oxygen therapy only during ac-tivity. Others may require hospitalization and
endotracheal in-tubation. If the patient has underlying coronary artery
disease, coronary artery revascularization with percutaneous translumi-nal
coronary angioplasty (PTCA) or bypass surgery
may be considered. If the patient’s condition is unresponsive to
advanced aggressive medical therapy, innovative therapies, in-cluding
mechanical assist devices and transplantation, may be considered.
Cardiac
resynchronization, involving the use of left ventricu-lar and biventricular
pacing, is a treatment for HF with electrical conduction defects. Left bundle
branch block (LBBB) is fre-quently found in patients with systolic dysfunction.
LBBB occurs when the electrical impulse, which normally depolarizes the right
and left bundle branches at the same time, depolarizes the right bundle branch
but not the left bundle branch. The dyssynchro-nous electrical stimulation of
the ventricles causes the right ven-tricle to contract before the left
ventricle, which can lead to further decreased ejection fraction (Gerber et
al., 2001). Use of a pacing device (eg, Medtronic InSync), with leads placed on
the inner wall of the right atrium and right ventricle and on the outer wall of
the left ventricle, provides synchronized electrical stimu-lation to the heart.
In one study, 63% of the patients who had re-ceived these devices showed
improvement in clinical status, including NYHA functional class and global
assessment, com-pared with 38% of placebo patients (Abraham, 2002).
PHARMACOLOGIC THERAPY
Several
medications are indicated for systolic HF. Medications for diastolic failure
depend on the underlying condition, such as hypertension or valvular dysfunction.
If the patient is in mild systolic failure, an ACE inhibitor usually is
prescribed. If the patient is unable to continue an ACE inhibitor (eg, because
of development of renal impairment as evidenced by elevated serum creatinine or
persistent serum potassium levels of 5.5 mEq/L or above), an angiotensin II
receptor blocker (ARB) or hydralazine and isosorbide dinitrate are considered
as part of the treatment plan. A diuretic is added if signs of fluid overload
develop. Digitalis is added to ACE inhibitors if the symptoms continue.
Although previously contraindicated in HF, specific beta-blockers decrease
mortality and morbidity if added to the initial medications. Spironolactone, a
weak diuretic may also be added for persistent symptoms.
Angiotensin-Converting Enzyme Inhibitors.
ACE inhibitors(ACE-Is) have a pivotal role in the management of HF due
to sys-tolic dysfunction. They have been found to relieve the signs and
symptoms of HF and significantly decrease mortality and mor-bidity (when used
to treat a symptomatic patient) by inhibiting neurohormonal activation
(CONSENSUS Trial Study Group, 1987; SOLVD Investigators, 1992). Available as
oral and intra-venous medications, ACE-Is promote vasodilation and diuresis by
decreasing afterload and preload. By doing so, they decrease the workload of
the heart. Vasodilation reduces resistance to left ven-tricular ejection of
blood, diminishing the heart’s workload and improving ventricular emptying. In
promoting diuresis, ACE-Is decrease the secretion of aldosterone, a hormone
that causes the kidneys to retain sodium. ACE-Is stimulate the kidneys to
excrete sodium and fluid (while retaining potassium), thereby reducing left
ventricular filling pressure and decreasing pulmonary congestion. ACE-Is may be
the first medication prescribed for patients in mild failure—patients with
fatigue or dyspnea on exertion but without signs of fluid overload and
pulmonary congestion.
Results
from studies (Clement et al., 2000; NETWORK Investigators, 1998) to identify
the specific dose to achieve this effect are equivocal, although one large
study showed significant reductions in death and hospitalization with higher
doses (Packer et al., 1999). However, it is recommended to start at a low dose
and increase every 2 weeks until the optimal dose is achieved and the patient
is hemodynamically stable. The final maintenance dose depends on the patient’s
blood pressure, fluid status, renal status, and degree of cardiac failure.
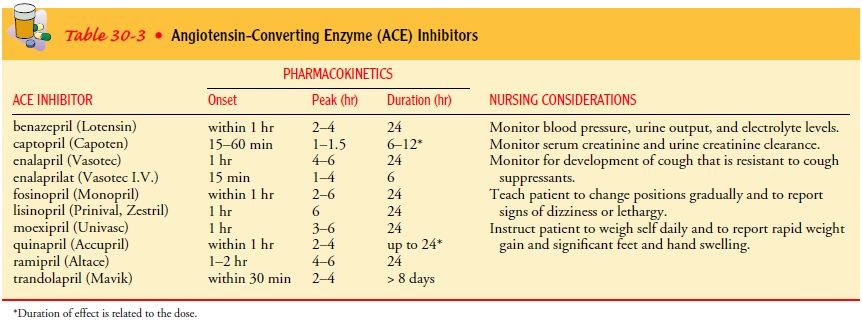
Patients
receiving ACE-I therapy are monitored for hypoten-sion, hypovolemia,
hyponatremia, and alterations in renal func-tion, especially if they are also
receiving diuretics. When to observe for these effects and for how long depends
on the onset, peak, and duration of the medication. Table 30-3 identifies
several types of ACE-Is and their pharmacokinetics. Hypotension is most likely
to develop from ACE-I therapy in patients older than age 75 and in those with a
systolic blood pressure of 100 mm Hg or less, a serum sodium level of less than
135 mEq/L, or severe cardiac fail-ure. Adjusting the dose or type of diuretic
in response to the pa-tient’s blood pressure and renal function may allow for
continued increases in the dosage of ACE-Is.
Because
ACE-Is cause the kidneys to retain potassium, the patient who is also receiving
a diuretic may not need to take oral potassium supplements. However, patients
receiving potassium-sparing diuretics (which do not cause potassium loss with
diuresis) must be carefully monitored for hyperkalemia, an increased level of
potassium in the blood. Before the initiation of the ACE-I, hy-perkalemic and
hypovolemic states must be corrected. ACE-Is may be discontinued if the
potassium remains above 5.0 mEq/L or if the serum creatinine is 3.0 mg/dL and
continues to increase. Other side effects of ACE-Is include a dry, persistent
cough that may not respond to cough suppressants. However, the cough could also
in-dicate a worsening of ventricular function and failure. Rarely, the cough
indicates angioedema. If angioedema affects the oropha-ryngeal area and impairs
breathing, the ACE-I must be stopped immediately.
Angiotensin II Receptor Blockers (ARBs).
Although their actionis different than that of ACE-Is, ARBs (eg,
losartan [Cozaar]) have a similar hemodynamic effect as ACE-Is: lowered blood
pressure and lowered systemic vascular resistance. Whereas ACE-Is block the
conversion of angiotensin I to angiotensin II, ARBs block the effects of
angiotensin II at the angiotensin II receptor. ACE-Is and ARBs also have
similar side effects: hyperkalemia, hypoten-sion, and renal dysfunction. ARBs
are usually prescribed when patients are not able to tolerate ACE-Is.
Hydralazine and Isosorbide Dinitrate.
A
combination of hy-dralazine (Apresoline) and isosorbide dinitrate
(Dilatrate-SR, Isordil, Sorbitrate) may be another alternative for patients who
cannot take ACE-Is. Nitrates (eg, isosorbide dinitrate) cause venous dilation, which
reduces the amount of blood return to the heart and lowers preload. Hydralazine
lowers systemic vascular resistance and left ventricular afterload. It has also
been shown to help avoid the development of nitrate tolerance. As with ARBs,
this combination of medications is usually used when patients are not able to
tolerate ACE-Is.
Beta-Blockers.
When used
with ACE-Is, beta-blockers, such ascarvedilol (Coreg), metoprolol (Lopressor,
Toprol), or bisopro-lol (Zebeta), have been found to reduce mortality and
morbidity in NYHA class II or III HF patients by reducing the cytotoxic
ef-fects from the constant stimulation of the sympathetic nervous system
(Beta-Blocker Evaluation of Survival Trial [BEST] Inves-tigators, 2001;
CIBIS-II Investigators and Committees, 1999; MERIT, 1999; Packer et al., 1996;
Packer et al., 2001). These agents have also been recommended for patients with
asympto-matic systolic dysfunction, such as after acute myocardial infarc-tion
or revascularization to prevent the onset of symptoms of HF. However,
beta-blockers may also produce many side effects, in-cluding exacerbation of
HF. The side effects are most common in the initial few weeks of treatment. The
most frequent side effects are dizziness, hypotension, and bradycardia. To
minimize these side effects, staggering the administration of the beta-blocker
with the ACE-I is recommended. Because of the side effects, beta-blockers are
initiated only after stabilizing the patient and ensuring a euvolemic (normal
volume) state. They are titrated slowly (every 2 weeks), with close monitoring
at each increase in dose. If the patient develops symptoms during the titration
phase, treat-ment options include increasing the diuretic, reducing the dose of
ACE-I, or decreasing the dose of the beta-blocker.
An important nursing role during titration is educating the pa-tient about the potential worsening of symptoms during the early phase of treatment, and that improvement may take several weeks. It is very important that nurses provide support to patients going through this symptom-provoking phase of treatment. Because beta-blockade can cause bronchiole constriction, a beta1-selective beta-blocker (ie, one that primarily blocks the beta-adrenergic re-ceptor sites in the heart), such as metoprolol (Lopressor, Toprol), is recommended for patients with well-controlled, mild to moder-ate asthma. However, these patients need to be monitored closely for increased asthma symptoms. Any type of beta-blocker is con-traindicated in patients with severe or uncontrolled asthma.
Diuretics.
Diuretics are medications used to
increase the rate ofurine production and the removal of excess extracellular
fluid from the body. Of the types of diuretics prescribed for patients with
edema from HF, three are most common: thiazide, loop, and potassium-sparing
diuretics. These medications are classified according to their site of action
in the kidney and their effects on renal electrolyte excretion and
reabsorption. Thiazide diuretics, such as metolazone (Mykrox, Zaroxolyn),
inhibit sodium and chloride reabsorption mainly in the early distal tubules.
They also increase potassium and bicarbonate excretion. Loop diuretics, such as
furosemide (Lasix), inhibit sodium and chloride reabsorption mainly in the
ascending loop of Henle. Patients with signs and symptoms of fluid overload
should be started on a diuretic, a thiazide for those with mild symptoms or a
loop diuretic for patients with more severe symptoms or with renal
insufficiency (Brater, 1998). Both types of diuretics may be used for those in
severe HF and
unresponsive to a single diuretic. These medications may not be necessary if
the patient responds to activity recommendations, avoidance of excessive fluid
intake (<2 quarts/day), and a low-sodium diet (eg, <2 g/day).
Spironolactone
(Aldactone) is a potassium-sparing diuretic that inhibits sodium reabsorption
in the late distal tubule and collect-ing duct. It has been found to be
effective in reducing mortality and morbidity in NYHA class III and IV HF
patients when added to ACE-Is, loop diuretics, and digoxin. Serum creatinine
and potas-sium levels are monitored frequently (eg, within the first week and
then every 4 weeks) when this medication is first administered.
Side effects of diuretics include electrolyte imbalances, symp-tomatic hypotension (especially with overdiuresis), hyperuricemia (causing gout), and ototoxicity. Dosages depend on the indica-tions, patient age, clinical signs and symptoms, and renal function. Table 30-4 lists commonly used diuretics, dosages, and pharma cokinetic properties.
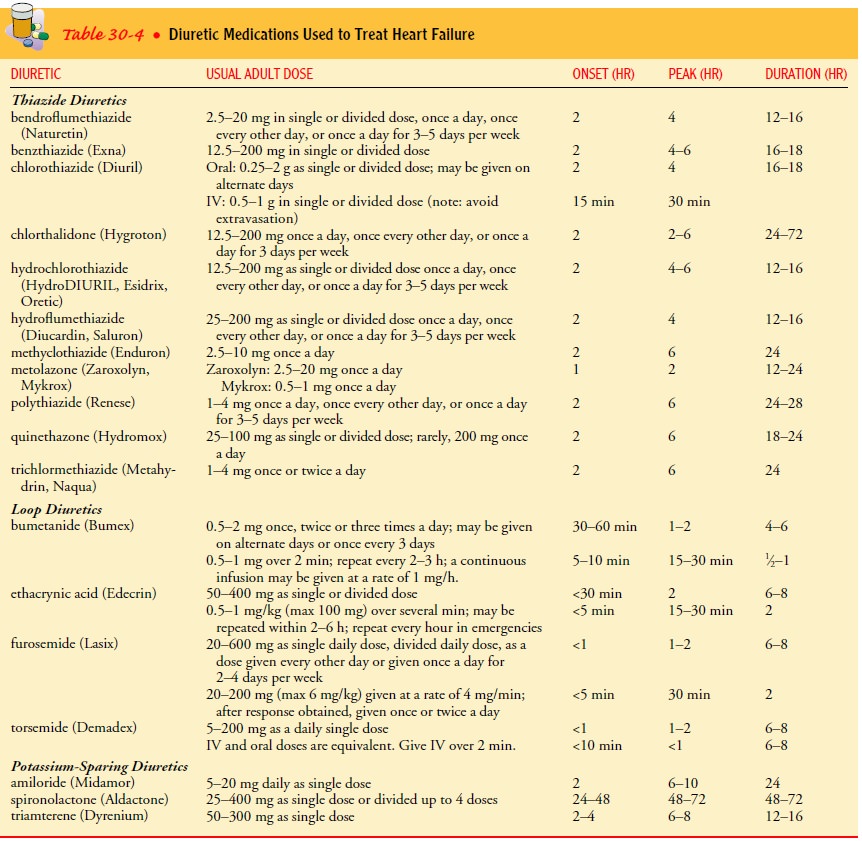
Careful patient monitoring and dose adjust-ments are necessary to
balance the effectiveness with the side ef-fects of therapy. Diuretics greatly
improve the patient’s symptoms, but they do not prolong life.
Digitalis.
The most commonly prescribed form
of digitalis for pa-tients with HF is digoxin (Lanoxin). The medication
increases the force of myocardial contraction and slows conduction through the
AV node. It improves contractility, increasing left ventricular output. The
medication also enhances diuresis, which removes fluid and relieves edema. The
effect of a given dose of medication depends on the state of the myocardium,
electrolyte and fluid balance, and renal and hepatic function. Although
digitalis does not decrease the mortality rate, it is effective in decreasing
the symptoms of systolic HF and in increasing the patient’s ability to perform
activities of daily living (Digitalis Investigation Group, 1997). It also has
been shown to significantly decrease hospital-ization rates and emergency room
visits for NYHA class II and III HF patients (Uretsky et al., 1993).
A key concern associated with digitalis therapy is digitalis tox-icity. Chart 30-3 summarizes the actions and uses of digitalis along with the nursing surveillance required when it is administered. The patient is observed for the effectiveness of digitalis ther-apy: lessening dyspnea and orthopnea, decrease in pulmonary crackles on auscultation, relief of peripheral edema, weight loss, and increase in activity tolerance.

The serum potassium level is mea-sured at intervals because
diuresis may have caused hypokalemia. The effect of digitalis is enhanced in
the presence of hypokalemia, so digitalis toxicity may occur. Serum digoxin
levels are obtained once each year or more frequently if there have been
changes in the patient’s medications, renal function, or symptoms.
Calcium Channel Blockers.
First-generation
calcium channelblockers, such as verapamil (Calan, Isoptin, Verelan),
nifedipine (Adalat, Procardia), and diltiazem (Cardizem, Dilacor, Tiazac), are
contraindicated in patients with systolic dysfunction, although they may be
used in patients with diastolic dysfunction. Am-lodipine (Norvasc) and
felodipine (Plendil), dihydropyridine calcium channel blockers, cause
vasodilation, reducing systemic vascular resistance. They may be used to
improve symptoms es-pecially in patients with nonischemic cardiomyopathy,
although they have no effect on mortality.
Other Medications.
Anticoagulants
may be prescribed, especiallyif the patient has a history of an embolic event
or atrial fibrillation or mural thrombus is present. Other medications such as
anti-anginal medications may be given to treat the underlying cause of HF.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprophen (Aleve,
Advil, Motrin) should be avoided. They can increase systemic vascular
resistance and decrease renal perfusion, especially in the elderly. For similar
rea-sons, use of decongestants should be avoided.
NUTRITIONAL THERAPY
A
low-sodium (≤ 2 to 3 g/day) diet and avoidance of excessive
amounts of fluid are usually recommended. Although it has not been shown to
affect the mortality rate, this recommendation re-duces fluid retention and the
symptoms of peripheral and pul-monary congestion. The purpose of sodium
restriction is to decrease the amount of circulating volume, which would
decrease the need for the heart to pump that volume. A balance needs to be
achieved between the ability of the patient to alter the diet and the amount of
medications that are prescribed. Any change in diet needs to be done with
consideration of good nutrition as well as the patient’s likes, dislikes, and
cultural food patterns.
Nursing Management
The
nurse is responsible for administering the medications and for assessing their
beneficial and detrimental effects to the patient. It is the balance of these
effects that determines the type and dosage of pharmacologic therapy. Nursing
actions to evaluate therapeutic effectiveness include the following:
· Keeping an intake and
output record to identify a negative balance (more output than input)
· Weighing the patient
daily at the same time and on the same scale, usually in the morning after
urination; moni-toring for a 2- to 3-lb gain in a day or 5-lb gain in week
· Auscultating lung sounds
at least daily to detect an increase or decrease in pulmonary crackles
· Determining the degree
of JVD
· Identifying and
evaluating the severity of dependent edema
· Monitoring pulse rate
and blood pressure, as well as moni-toring for postural hypotension and making
sure that the patient does not become hypotensive from dehydration
· Examining skin turgor
and mucous membranes for signs of dehydration
· Assessing symptoms of
fluid overload (eg, orthopnea, parox-ysmal nocturnal dyspnea, and dyspnea on
exertion) and evaluating changes
MONITORING AND MANAGING POTENTIAL COMPLICATIONS
Profuse
and repeated diuresis can lead to hypokalemia (ie, potas-sium depletion). Signs
are weak pulse, faint heart sounds, hypo-tension, muscle flabbiness, diminished
deep tendon reflexes, and generalized weakness. Hypokalemia poses new problems
for the pa-tient with HF because it markedly weakens cardiac contractions. In
patients receiving digoxin, hypokalemia can lead to digitalis tox-icity.
Digitalis toxicity and hypokalemia increase the likelihood of dangerous
dysrhythmias (see Chart 30-3). Low levels of potassium may also indicate a low
level of magnesium, which can add to the risk for dysrhythmias. Hyperkalemia
may also occur, especially with the use of ACE-Is or ARBs and spironolactone.
Prolonged
diuretic therapy may also produce hyponatremia (deficiency of sodium in the
blood), which results in apprehen-sion, weakness, fatigue, malaise, muscle
cramps and twitching, and a rapid, thready pulse.
Other
problems associated with diuretic administration are hyperuricemia (excessive
uric acid in the blood), volume deple-tion from excessive urination, and
hyperglycemia.
Gerontologic Considerations
Several normal changes that occur with aging
increase the frequency of diastolic HF: increased systolic blood pressure,
increased ventric ular wall thickness, increased atrial size, and increased
myocardial fi-brosis. Elderly people may present with atypical signs and
symptoms: fatigue, weakness, and somnolence. Decreased renal function makes the
elderly patient resistant to diuretics and more sensitive to changes in volume,
especially with diastolic dysfunction. The ad-ministration of diuretics to
elderly men requires nursing surveil-lance for bladder distention caused by
urethral obstruction from an enlarged prostate gland. The bladder may be
assessed with an ul-trasound scanner, or the suprapubic area palpated for an
oval mass and percussed for dullness, indicative of bladder fullness,
Related Topics