Chapter: Basic & Clinical Pharmacology : Beta-Lactam & OtherCell Wall- & Membrane-Active Antibiotics
Cephalosporins & Cephamycins
CEPHALOSPORINS & CEPHAMYCINS
Cephalosporins are
similar to penicillins, but more stable to many bacterial β lactamases and
therefore have a broader spectrum of activity. However, strains of E coli and Klebsiella sp expressing extended-spectrum β lactamases that can hydrolyze most
cepha-losporins are a growing clinical concern. Cephalosporins are not active
against enterococci and L monocytogenes.
Chemistry
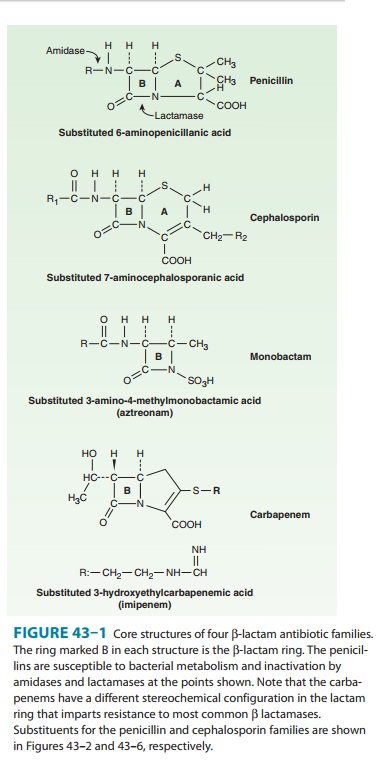
The
nucleus of the cephalosporins, 7-aminocephalosporanic acid (Figure 43–6), bears
a close resemblance to 6-aminopenicillanic acid (Figure 43–1). The intrinsic
antimicrobial activity of natural cephalosporins is low, but the attachment of
various R1 and R2
groups has yielded hundreds of potent compounds of low toxicity. Cephalosporins
can be classified into four major groups or gen-erations, depending mainly on
the spectrum of antimicrobial activity.
FIRST-GENERATION CEPHALOSPORINS
First-generation
cephalosporins include cefazolin,
cefadroxil,cephalexin, cephalothin, cephapirin, and cephradine. Thesedrugs are very active against gram-positive
cocci, such as pneumo-cocci, streptococci, and staphylococci. Traditional
cephalosporins are not active against methicillin-resistant strains of
staphylococci; how-ever, new compounds have been developed that have activity
against methicillin-resistant strains . E
coli, K pneumoniae, and Proteus
mirabilis are often sensitive, but activity against
P
aeruginosa,
indole-positive proteus species, Enterobacter
sp,S marcescens, Citrobacter sp, and Acinetobacter sp is poor. Anaerobiccocci (eg, peptococci,
peptostreptococci) are usually sensitive, but Bacteroides fragilis is not.
Pharmacokinetics & Dosage
A. Oral
Cephalexin,
cephradine, and cefadroxil are absorbed from the gut to a variable extent.
After oral doses of 500 mg, serum levels are 15–20 mcg/mL. Urine concentration
is usually very high, but in most tis-sues levels are variable and generally
lower than in serum. Cephalexin and cephradine are given orally in dosages of
0.25–0.5 g four times daily (15–30 mg/kg/d) and cefadroxil in dosages of 0.5–1
g twice daily. Excretion is mainly by glomerular filtration and tubular
secre-tion into the urine. Drugs that block tubular secretion, eg, probenecid,
may increase serum levels substantially. In patients with impaired renal
function, dosage must be reduced (Table 43–2).
B. Parenteral
Cefazolin
is the only first-generation parenteral cephalosporin still in general use.
After an intravenous infusion of 1 g, the peak level of cefazolin is 90–120
mcg/mL. The usual intravenous dosage of cefazolin for adults is 0.5–2 g
intravenously every 8 hours. Cefazolin can also be administered
intramuscularly. Excretion is via the kidney, and dose adjustments must be made
for impaired renal function.
Clinical Uses
Oral
drugs may be used for the treatment of urinary tract infec-tions and
staphylococcal or streptococcal infections, including cellulitis or soft tissue
abscess. However, oral cephalosporins should not be relied on in serious
systemic infections.
Cefazolin
penetrates well into most tissues. It is a drug of choice for surgical
prophylaxis. Cefazolin may also be a choice in infections for which it is the
least toxic drug (eg, penicillinase-producing E coli or K pneumoniae)
and in individuals with staphylococcal or streptococcal infections who have a
history of penicillin allergy other than immediate hypersensitivity. Cefazolin
does not penetrate the central nervous system and cannot be used to treat
meningitis. Cefazolin is an alternative to an antistaphylo-coccal penicillin
for patients who are allergic to penicillin.
SECOND-GENERATION CEPHALOSPORINS
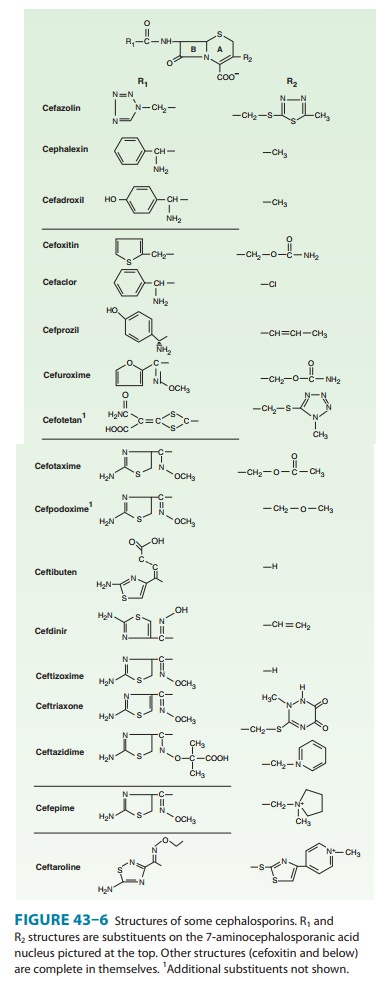
Members of the
second-generation cephalosporins include cefaclor,cefamandole,
cefonicid, cefuroxime, cefprozil, loracarbef, and ceforanide; and the structurally related cephamycins cefoxitin, cefmetazole, and cefotetan, which have activity against
anaer-obes. This is a heterogeneous group with marked individual differ-ences
in activity, pharmacokinetics, and toxicity. In general, they are active
against organisms inhibited by first-generation drugs, but in addition they
have extended gram-negative coverage. Klebsiella
sp (including those resistant to cephalothin) are usuallysensitive.
Cefamandole, cefuroxime, cefonicid, ceforanide, and cefaclor are active against
H influenzae but not against serratia
or B fragilis. In contrast,
cefoxitin, cefmetazole, and cefotetan areactive against B fragilis and some serratia strains but are less active against H influenzae. As with first-generation
agents, none is active against enterococci or P aeruginosa. Second-generation cepha-losporins may exhibit in
vitro activity against Enterobacter
sp., but resistant mutants that constitutively express a chromosomal lactamase
that hydrolyzes these compounds (and third-generation cephalosporins) are readily
selected, and they should not be used to treat enterobacter infections.
Pharmacokinetics & Dosage
A. Oral
Cefaclor,
cefuroxime axetil, cefprozil, and loracarbef can be given orally. The usual
dosage for adults is 10–15 mg/kg/d in two to four divided doses; children
should be given 20–40 mg/kg/d up to a maximum of 1 g/d. Except for cefuroxime
axetil, these drugs are not predictably active against
penicillin-non-susceptible pneumo-cocci and should be used cautiously, if at
all, to treat suspected or proved pneumococcal infections. Cefaclor is more
susceptible to β-lactamase
hydrolysis compared with the other agents, and itsusefulness is correspondingly
diminished.
B. Parenteral
After
a 1-g intravenous infusion, serum levels are 75–125 mcg/mL for most second-generation
cephalosporins. Intramuscular admin-istration is painful and should be avoided.
Doses and dosing intervals vary depending on the specific agent (Table 43–2).
There are marked differences in half-life, protein binding, and interval
between doses. All are renally cleared and require dosage adjust-ment in renal
failure.
Clinical Uses
The
oral second-generation cephalosporins are active against β-lactamase-producingH influenzaeorMoraxella catarrhalisand havebeen primarily used to treat
sinusitis, otitis, and lower respiratory tract infections, in which these
organisms have an important role. Because of their activity against anaerobes
(including many B fragilis strains),
cefoxitin, cefotetan, or cefmetazole can be used to treat mixed anaero-bic
infections such as peritonitis, diverticulitis, and pelvic inflamma-tory
disease. Cefuroxime is used to treat community-acquired pneumonia because it is
active against β-lactamase-producing
H influenzae or K pneumoniae and some penicillin-non-susceptiblepneumococci.
Although cefuroxime crosses the blood-brain barrier, it is less effective in
treatment of meningitis than ceftriaxone or cefo-taxime and should not be used.
THIRD-GENERATION CEPHALOSPORINS
Third-generation
agents include cefoperazone, cefotaxime,
cef-tazidime, ceftizoxime, ceftriaxone, cefixime, cefpodoxime prox-etil,
cefdinir, cefditoren pivoxil, ceftibuten, and moxalactam.
Antimicrobial Activity
Compared
with second-generation agents, these drugs have expanded gram-negative
coverage, and some are able to cross the blood-brain barrier. Third-generation
drugs are active against Citrobacter, S marcescens, and Providencia (although resistance canemerge during treatment of
infections caused by these species due to selection of mutants that
constitutively produce cephalosporinase). They are also effective against β-lactamase-producing
strains of haemophilus and neisseria. Ceftazidime and cefoperazone are the only
two drugs with useful activity against P
aeruginosa. Like the second-generation drugs, third-generation
cephalosporins are hydrolyzed by constitutively produced AmpC β lactamase,
and they are not reliably active against Enterobacter
species. Serratia, Providencia, and Citrobacter also produce a chromosomallyencoded cephalosporinase
that, when constitutively expressed, can confer resistance to third-generation
cephalosporins. Ceftizoxime and moxalactam are active against B fragilis. Cefixime, cefdinir,ceftibuten,
and cefpodoxime proxetil are oral agents possessing similar activity except
that cefixime and ceftibuten are much less active against pneumococci and have
poor activity against S aureus.
Pharmacokinetics & Dosage
Intravenous infusion
of 1 g of a parenteral cephalosporin produces serum levels of 60–140 mcg/mL.
Third-generation cephalosporins penetrate body fluids and tissues well and,
with the exception of cefoperazone and all oral cephalosporins, achieve levels
in the cerebrospinal fluid sufficient to inhibit most susceptible pathogens.
The half-lives of
these drugs and the necessary dosing intervals vary greatly: Ceftriaxone
(half-life 7–8 hours) can be injected once every 24 hours at a dosage of 15–50
mg/kg/d. A single daily 1-g dose is sufficient for most serious infections,
with 2 g every 12 hours recommended for treatment of meningitis. Cefoperazone
(half-life 2 hours) can be infused every 8–12 hours in a dosage of 25–100
mg/kg/d. The remaining drugs in the group (half-life 1–1.7 hours) can be infused
every 6–8 hours in dosages between 2 and 12 g/d, depending on the severity of
infection. Cefixime can be given orally (200 mg twice daily or 400 mg once
daily) for urinary tract infections and as a single 400 mg dose for
uncompli-cated gonococcal urethritis and cervicitis. The adult dose for
cefpodoxime proxetil or cefditoren pivoxil is 200–400 mg twice daily; for
ceftibuten, 400 mg once daily; and for cefdinir, 300 mg/12 h. The excretion of
cefoperazone and ceftriaxone is mainly through the biliary tract, and no dosage
adjustment is required in renal insufficiency. The others are excreted by the
kidney and therefore require dosage adjustment in renal insufficiency.
Clinical Uses
Third-generation
cephalosporins are used to treat a wide variety of serious infections caused by
organisms that are resistant to most other drugs. Strains expressing
extended-spectrum β
lactamases, however, are not susceptible. Third-generation cephalosporins
should be avoided in treatment of enterobacter infections—even if the clinical
isolate appears susceptible in vitro—because of emergence of resistance.
Ceftriaxone and cefotaxime are approved for treatment of meningitis, including
meningitis caused by pneu-mococci, meningococci, H influenzae, and susceptible enteric gram-negative rods, but not
by L monocytogenes. Ceftriaxone and
cefotaxime are the most active cephalosporins against
penicillin-non-susceptible strains of pneumococci and are recommended for
empirical therapy of serious infections that may be caused by these strains.
Meningitis caused by strains of pneumococci with penicil-lin MICs > 1 mcg/mL may not
respond even to these agents, and addition of vancomycin is recommended. Other
potential indica-tions include empirical therapy of sepsis of unknown cause in
both the immunocompetent and the immunocompromised patient and treatment of
infections for which a cephalosporin is the least toxic drug available. In
neutropenic, febrile immunocom-promised patients, ceftazidime is often used in
combination with other antibiotics.
FOURTH-GENERATION CEPHALOSPORINS
Cefepime
is an example of a so-called fourth-generation cephalosporin. It is more
resistant to hydrolysis by chromosomallactamases (eg, those produced by Enterobacter). However, like the
third-generation compounds, it is hydrolyzed by extended
spectrum β lactamases. Cefepime has good activity against aeruginosa, Enterobacteriaceae,
S aureus, and S pneumoniae. Itis
highly active against Haemophilus and
Neisseria sp. It penetrates well into
cerebrospinal fluid. It is cleared by the kidneys and has a half-life of 2
hours, and its pharmacokinetic properties are very similar to those of
ceftazidime. Unlike ceftazidime, however, cefepime has good activity against
most penicillin-non-susceptible strains of streptococci, and it is useful in
treatment of entero-bacter infections.
Cephalosporins Active against Methicillin-Resistant Staphylococci
Beta-lactam
antibiotics with activity against methicillin-resistant staphylococci are
currently under development. Ceftaroline
fosamil, the prodrug of the active metabolite ceftaroline, is the first such
drug to be approved for clinical use in the USA. Ceftaroline has increased
binding to penicillin-binding protein 2a, which mediates methicillin resistance
in staphylococci, resulting in bactericidal activity against these strains. It
has some activity against enterococci and a broad gram-negative spectrum,
although it is not active against extended-spectrum β-lactamase-producing strains. Since clinical
experience with this and similar investiga-tional drugs is limited, their role
in therapy is not yet defined.
ADVERSE EFFECTS OF CEPHALOSPORINS
A. Allergy
Cephalosporins
are sensitizing and may elicit a variety of hyper-sensitivity reactions that
are identical to those of penicillins, including anaphylaxis, fever, skin
rashes, nephritis, granulocy-topenia, and hemolytic anemia. However, the
chemical nucleus of cephalosporins is sufficiently different from that of
penicillins so that some individuals with a history of penicillin allergy may
tolerate cephalosporins. The frequency of cross-allergenicity between the two
groups of drugs is uncertain but is probably around 5–10%. Cross-allergenicity
appears to be more common with penicillins and early generation cephalosporins
compared with later generation cephalosporins. However, patients with a history
of anaphylaxis to penicillins should not receive cephalosporins.
B. Toxicity
Local
irritation can produce pain after intramuscular injection and thrombophlebitis
after intravenous injection. Renal toxicity, including interstitial nephritis
and tubular necrosis, has been dem-onstrated with several cephalosporins and
caused the withdrawal of cephaloridine from clinical use.
Cephalosporins that
contain a methylthiotetrazole group (cefa-mandole, cefmetazole, cefotetan, and
cefoperazone) may cause hypoprothrombinemia and bleeding disorders. Oral
administra-tion of vitamin K1, 10 mg twice weekly, can prevent this. Drugs with the
methylthiotetrazole ring can also cause severe disulfiram-like reactions;
consequently, alcohol and alcohol-containing medications must be avoided.
Related Topics