Chapter: Basic & Clinical Pharmacology : Beta-Lactam & OtherCell Wall- & Membrane-Active Antibiotics
Beta Lactam Compounds: Penicillins
BETA LACTAM COMPOUNDS
PENICILLINS
The penicillins share
features of chemistry, mechanism of action, pharmacology, and immunologic
characteristics with cephalosporins, monobactams, carbapenems, and β-lactamase inhibitors.
All are β-lactam
compounds, so named because of their four-memberedlactam ring.
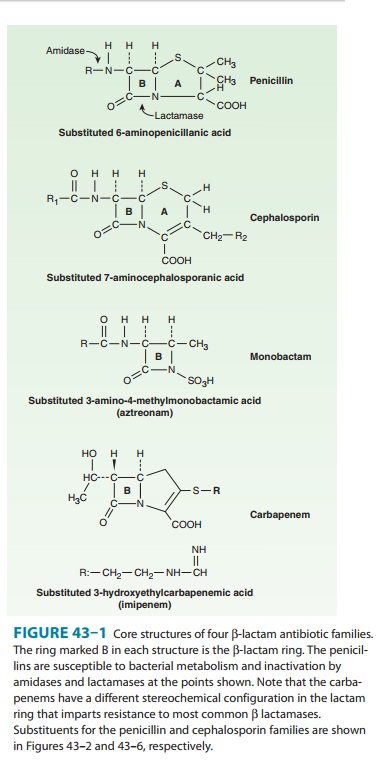
Chemistry
All penicillins have
the basic structure shown in Figure 43–1. A thiazolidine ring (A) is attached
to a β-lactam
ring (B) that carries a secondary amino group (RNH–). Substituents (R; examples
shown in Figure 43–2) can be attached to the amino group. Structural integrity
of the 6-aminopenicillanic acid nucleus (rings A plus B) is essential for the
biologic activity of these compounds.Hydrolysis of the β-lactam ring by bacterial β-lactamases yields
penicilloic acid, which lacks antibacterial activity.
A. Classification
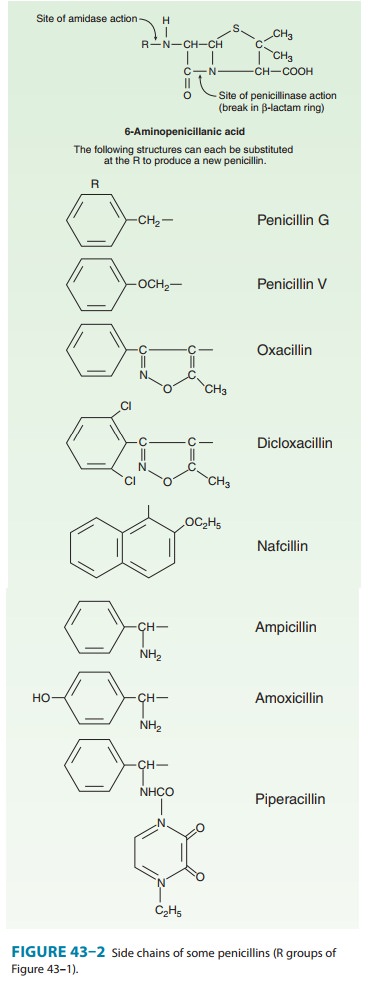
Substituents
of the 6-aminopenicillanic acid moiety determine the essential pharmacologic
and antibacterial properties of the result-ing molecules. Penicillins can be
assigned to one of three groups (below). Within each of these groups are
compounds that are rela-tively stable to gastric acid and suitable for oral
administration, eg, penicillin V, dicloxacillin, and amoxicillin. The side chains
of some representatives of each group are shown in Figure 43–2, with a few
distinguishing characteristics.
1. Penicillins (eg, penicillin
G)—These
have greatest activityagainst gram-positive organisms, gram-negative cocci, and
non-β-lactamase
producing anaerobes. However, they have little activ-ity against gram-negative
rods, and they are susceptible to hydrolysis by β-lactamases.
2. Antistaphylococcal penicillins (eg, nafcillin)—Thesepenicillins are resistant to staphylococcal β-lactamases. They are active against staphylococci and streptococci but not against enterococci, anaerobic bacteria, and gram-negative cocci and rods.
3. Extended-spectrum penicillins
(ampicillin and the antipseudomonal penicillins)— These drugs retain the
anti-bacterial spectrum of penicillin and have improved activity
againstgram-negative organisms. Like penicillin, however, they are rela-tively
susceptible to hydrolysis by β-lactamases.
B. Penicillin Units and Formulations
The activity of penicillin G was originally defined in units. Crystalline sodium penicillin G contains approximately 1600 units per mg (1 unit = 0.6 mcg; 1 million units of penicillin = 0.6 g).
Semisynthetic penicillins are
prescribed by weight rather than units. The minimum inhibitory concentration (MIC) of any penicillin (or other
antimicrobial) is usually given in mcg/mL. Most penicillins are formulated as
the sodium or potassium salt of the free acid. Potassium penicillin G contains
about 1.7 mEq of K+ per million units of penicillin (2.8 mEq/g). Nafcillin contains
Na+, 2.8 mEq/g. Procaine
salts and benzathine salts of penicillin G provide repository forms for
intramuscular injection. In dry crystalline form, penicillin salts are stable
for years at 4°C.
Solutions lose their activity rapidly (eg, 24 hours at 20°C) and must be
prepared fresh for administration.
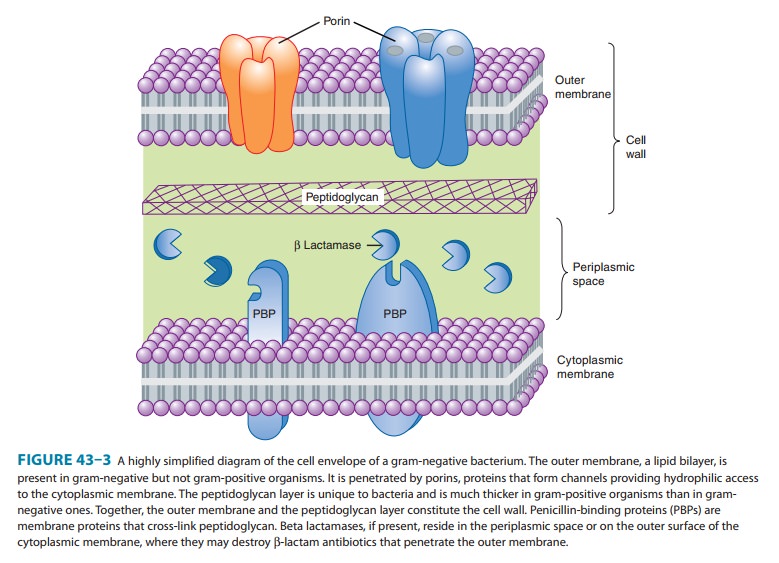
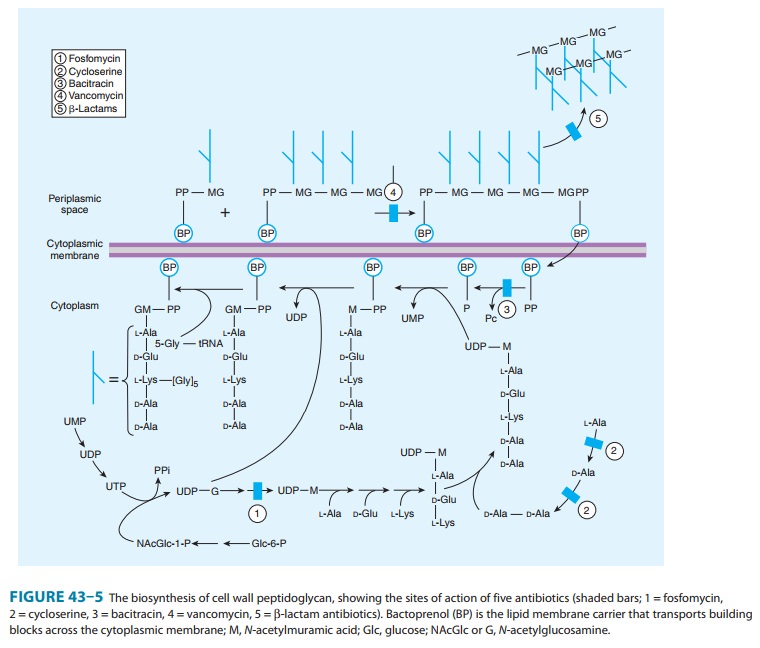
Mechanism of Action
Penicillins, like all β-lactam antibiotics,
inhibit bacterial growth by interfering with the transpeptidation reaction of bacterial cellwall synthesis. The cell
wall is a rigid outer layer unique to bacte-rial species. It completely
surrounds the cytoplasmic membrane (Figure 43–3), maintains cell shape and
integrity, and prevents cell lysis from high osmotic pressure. The cell wall is
composed of complex, cross-linked polymer of polysaccharides and polypeptides,
peptidoglycan (also known as murein or mucopep-tide). The polysaccharide
contains alternating amino sugars,
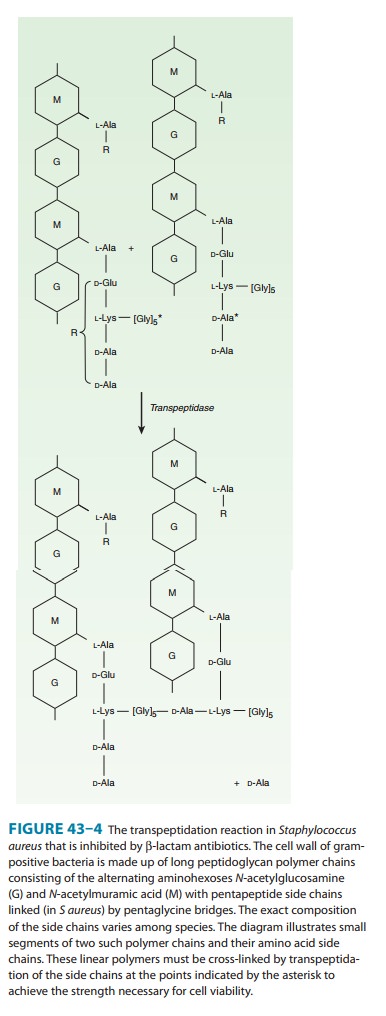
N-acetylglucosamine
and N-acetylmuramic acid (Figure
43–4). Afive-amino-acid peptide is linked to the N-acetylmuramic acid sugar. This peptide terminates in D-alanyl-D-alanine.
Penicillin-binding protein (PBP, an enzyme) removes the terminal alanine in the
process of forming a cross-link with a nearby peptide. Cross-links give the
cell wall its structural rigidity. Beta-lactam antibiot-ics, structural analogs
of the natural D-Ala-D-Ala
substrate, covalently bind to the active site of PBPs. This inhibits the
trans-peptidation reaction (Figure 43–5), halting peptidoglycan synthe-sis, and
the cell dies. The exact mechanism of cell death is not completely understood,
but autolysins and disruption of cell wall morphogenesis are involved.
Beta-lactam antibiotics kill bacterial cells only when they are actively
growing and synthesizing cell wall.
Resistance
Resistance to
penicillins and other β-lactams is due to one of four general
mechanisms: (1) inactivation of antibiotic by β-lactamase,modification of target PBPs, (3)
impaired penetration of drug to target PBPs, and (4) efflux. Beta-lactamase
production is the most common mechanism of resistance. Hundreds of
different β-lactamases have been identified. Some, such as those produced by
Staphylococcus aureus, Haemophilus influenzae, and Escherichia coli,
Other β-lactamases, eg, AmpC β-lactamase pro-duced
by Pseudomonas aeruginosa and Enterobacter sp, and extended-spectrum β-lactamases (ESBLs),
hydrolyze both cephalosporins and penicillins. Carbapenems are highly resistant
to hydrolysis by peni-cillinases and cephalosporinases, but they are hydrolyzed
by metallo-β
lactamase and carbapenemases.
Altered target PBPs
are the basis of methicillin resistance in staphylococci and of penicillin
resistance in pneumococci and enterococci. These resistant organisms produce
PBPs that have low affinity for binding β-lactam antibiotics, and consequently, they
are not inhibited except at relatively high, often clinically unachievable,
drug concentrations.
Resistance due to
impaired penetration of antibiotic to target PBPs occurs only in gram-negative
species because of their imper-meable outer cell wall membrane, which is absent
in gram-positive bacteria. Beta-lactam antibiotics cross the outer membrane and
enter gram-negative organisms via outer membrane protein channels called
porins. Absence of the proper channel or down-regulation of its production can
greatly impair drug entry into the cell. Poor penetration alone is usually not
sufficient to confer resistance because enough antibiotic eventually enters the
cell to inhibit growth. However, this barrier can become important in the
presence of a β-lactamase,
even a relatively inactive one, as long as it can hydrolyze drug faster than it
enters the cell. Gram-negative organisms also may produce an efflux pump, which
consists of cytoplasmic and periplasmic protein components that efficiently
transport some β-lactam
antibiotics from the periplasm back across the outer membrane.
Pharmacokinetics
Absorption of orally
administered drug differs greatly for different penicillins, depending in part
on their acid stability and protein binding. Gastrointestinal absorption of
nafcillin is erratic, so it is not suitable for oral administration.
Dicloxacillin, ampicillin, and amoxicillin are acid-stable and relatively well
absorbed, producing serum concentrations in the range of 4–8 mcg/mL after a
500-mg oral dose. Absorption of most oral penicillins (amoxicillin being an
exception) is impaired by food, and the drugs should be admin-istered at least
1–2 hours before or after a meal.
Intravenous administration of penicillin G is preferred to the intramuscular route because of irritation and local pain from intra-muscular injection of large doses. Serum concentrations 30 minutes after an intravenous injection of 1 g of a penicillin (equivalent to approximately 1.6 million units of penicillin G) are 20–50 mcg/ mL. Only a small amount of the total drug in serum is present as free drug, the concentration of which is determined by protein binding. Highly protein-bound penicillins (eg, nafcillin) generally achieve lower free-drug concentrations in serum than less protein-bound penicillins (eg, penicillin G or ampicillin). Protein binding becomes clinically relevant when the protein-bound percentage is approximately 95% or more. Penicillins are widely distributed in body fluids and tissues with a few exceptions. They are polar mol-ecules, so intracellular concentrations are well below those found in extracellular fluids.
Benzathine and
procaine penicillins are formulated to delay absorption, resulting in prolonged
blood and tissue concentra-tions. A single intramuscular injection of 1.2
million units of benzathine penicillin maintains serum levels above 0.02 mcg/mL
for 10 days, sufficient to treat β-hemolytic streptococcal infection. After 3
weeks, levels still exceed 0.003 mcg/mL, which is enough to prevent β-hemolytic
streptococcal infection. A 600,000 unit dose of procaine penicillin yields peak
concentrations of 1–2 mcg/ mL and clinically useful concentrations for 12–24
hours after a single intramuscular injection.
Penicillin
concentrations in most tissues are equal to those in serum. Penicillin is also
excreted into sputum and milk to levels 3–15% of those in the serum.
Penetration into the eye, the prostate, and the central nervous system is poor.
However, with active inflammation of the meninges, as in bacterial meningitis,
penicillin concentrations of 1–5 mcg/mL can be achieved with a daily
par-enteral dose of 18–24 million units. These concentrationsare sufficient to
kill susceptible strains of pneumococci and meningococci.
Penicillin
is rapidly excreted by the kidneys; small amounts are excreted by other routes.
About 10% of renal excretion is by glomerular filtration and 90% by tubular
secretion. The normal half-life of penicillin G is approximately 30 minutes; in
renal failure, it may be as long as 10 hours. Ampicillin and the
extended-spectrum penicillins are secreted more slowly than penicillin G and
have half-lives of 1 hour. For penicillins that are cleared by the kidney, the
dose must be adjusted according to renal function, with approximately one
fourth to one third the normal dose being administered if creatinine clearance
is 10 mL/min or less (Table 43–1).
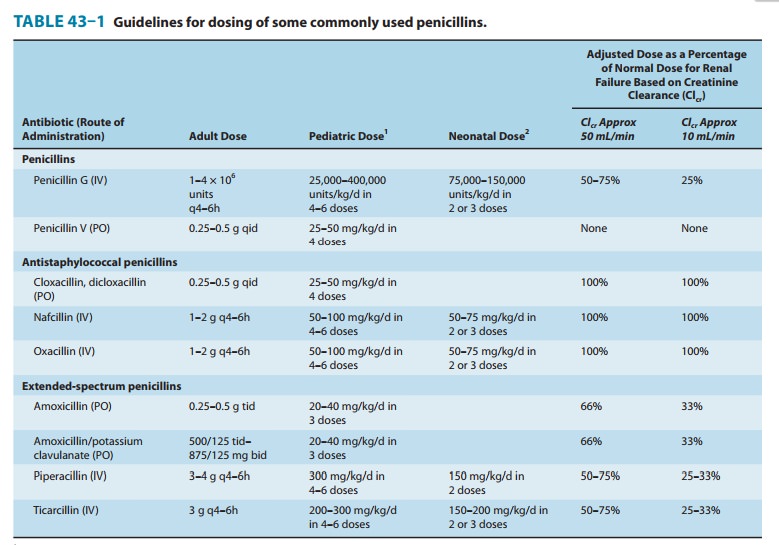
Nafcillin is primarily cleared by biliary excretion. Oxacillin, dicloxacillin, and cloxacillin are eliminated by both the kidney and biliary excretion; no dosage adjustment is required for these drugs in renal failure. Because clearance of penicillins is less efficient in the newborn, doses adjusted for weight alone result in higher sys-temic concentrations for longer periods than in the adult.
Clinical Uses
Except for oral
amoxicillin, penicillins should be given 1–2 hours before or after a meal; they
should not be given with food to minimize binding to food proteins and acid
inactivation. Blood levels of all penicillins can be raised by simultaneous
administra-tion of probenecid, 0.5 g (10 mg/kg in children) every 6 hours
orally, which impairs renal tubular secretion of weak acids such as β-lactam compounds.
Penicillins should never be used for viralinfections and should be prescribed
only when there is reasonable suspicion of, or documented infection with,
susceptible organisms.
A. Penicillin
Penicillin
G is a drug of choice for infections caused by streptococci, meningococci, some
enterococci, penicillin-susceptible pneumo-cocci, non-β-lactamase-producing staphylococci,
Treponemapallidum and certain other
spirochetes, Clostridium species,
Actinomyces and
certain other gram-positive rods, and non-β-lactamase-producing gram-negative
anaerobic organisms. Depend-ing on the organism, the site, and the severity of
infection, effective doses range between 4 and 24 million units per day
administered intravenously in four to six divided doses. High-dose penicillin G
can also be given as a continuous intravenous infusion.
Penicillin
V, the oral form of penicillin, is indicated only in minor infections because
of its relatively poor bioavailability, the need for dosing four times a day,
and its narrow antibacterial spectrum. Amoxicillin is often used instead.
Benzathine
penicillin and procaine penicillin G for intramus-cular injection yield low but
prolonged drug levels. A single intra-muscular injection of benzathine
penicillin, 1.2 million units, is effective treatment for β-hemolytic
streptococcal pharyngitis; given intramuscularly once every 3–4 weeks, it
prevents reinfec-tion. Benzathine penicillin G, 2.4 million units intramuscularly
once a week for 1–3 weeks, is effective in the treatment of syphilis. Procaine
penicillin G, formerly a work horse for treating uncom-plicated pneumococcal
pneumonia or gonorrhea, is rarely used now because many strains are
penicillin-resistant.
B. Penicillins Resistant to Staphylococcal Beta Lactamase (Methicillin, Nafcillin, and Isoxazolyl Penicillins)
These
semisynthetic penicillins are indicated for infection by β-lactamase-producing
staphylococci, although penicillin-susceptible strains of streptococci and
pneumococci are also suscep-tible to these agents. Listeria monocytogenes, enterococci, and methicillin-resistant
strains of staphylococci are resistant. In recent years the empirical use of
these drugs has decreased substantially because of increasing rates of
methicillin-resistance in staphylo-cocci. However, for infections caused by
methicillin-susceptible and penicillin-resistant strains of staphylococci, these
are consid-ered the drugs of choice.
An
isoxazolyl penicillin such as oxacillin, cloxacillin, or dicloxacil-lin,
0.25–0.5 g orally every 4–6 hours (15–25 mg/kg/d for children), is suitable for
treatment of mild to moderate localized staphylococcal infections. All are
relatively acid-stable and have reasonable bioavail-ability. However, food
interferes with absorption, and the drugs should be administered 1 hour before
or after meals.
For
serious systemic staphylococcal infections, oxacillin or nafcillin, 8–12 g/d,
is given by intermittent intravenous infusion of 1–2 g every 4–6 hours (50–100
mg/kg/d for children).
C. Extended-Spectrum Penicillins (Aminopenicillins, Carboxypenicillins, and Ureidopenicillins)
These
drugs have greater activity than penicillin against gram-negative bacteria
because of their enhanced ability to penetrate the gram-negative outer
membrane. Like penicillin G, they are inacti-vated by many β
lactamases.
The aminopenicillins,
ampicillin and amoxicillin, have nearly identical spectrums of actiity, but
amoxicillin is better absorbed orally. Amoxicillin, 250–500 mg three times
daily, is equivalent to the same amount of ampicillin given four times daily.
Amoxacillin is given orally to treat urinary tract infections, sinusitis,
otitis, and lower respiratory tract infections. Ampicillin and amoxicillin are
the most active of the oral β-lactam antibiotics against pneumo-cocci with
elevated MICs to penicillin and are the preferred β-lactam antibiotics for treating infections
suspected to be causedby these strains. Ampicillin (but not amoxicillin) is
effective for shigellosis. Its use to treat uncomplicated salmonella
gastroenteritis is controversial because it may prolong the carrier state.
Ampicillin,
at dosages of 4–12 g/d intravenously, is useful for treating serious infections
caused by susceptible organisms, includ-ing anaerobes, enterococci, L monocytogenes, and β-lactamase-negative
strains of gram-negative cocci and bacilli such as E coli, and Salmonella sp.
Non-β-lactamase
producing strains of H influenzae are
generally susceptible, but strains that are resistant because of altered PBPs
are emerging. Many gram-negative species produce β lactamases and are resistant,
precluding use of ampicillin for empir-ical therapy of urinary tract
infections, meningitis, and typhoid fever. Ampicillin is not active against Klebsiella sp, Enterobacter sp, P aeruginosa,
Citrobacter sp, Serratia marcescens, indole-posi-tive proteus species, and other
gram-negative aerobes that are commonly encountered in hospital-acquired
infections. These organisms produce β lactamase that inactivates
ampicillin.
Carbenicillin,
the first antipseudomonal carboxypenicillin, is no longer used in the USA, as
there are more active, better toler-ated alternatives. A carboxypenicillin with
activity similar to that of carbenicillin is ticarcillin. It is less active
than ampicillin against enterococci. The ureidopenicillins, piperacillin,
mezlocillin, and azlocillin, are also active against selected gram-negative
bacilli, such as Klebsiella pneumoniae.
Although supportive clinical data are lacking for superiority of combination
therapy over single-drug therapy, because of the propensity of P aeruginosa to develop resistance
during treatment, an antipseudomonal penicillin is fre-quently used in
combination with an aminoglycoside or fluoro-quinolone for pseudomonal infections
outside the urinary tract.
Ampicillin,
amoxicillin, ticarcillin, and piperacillin are also available in combination
with one of several β-lactamase
inhib-itors: clavulanic acid, sulbactam, or tazobactam. The addition of a β-lactamase
inhibitor extends the activity of these penicil-lins to include β-lactamase-producing
strains of S aureus as well as some β-lactamase-producing
gram-negative bacteria (see Beta-Lactamase Inhibitors).
Adverse Reactions
The penicillins are
generally well tolerated, and unfortunately, this encourages their misuse and
inappropriate use. Most of the serious adverse effects are due to
hypersensitivity. All penicillins are cross-sensitizing and cross-reacting. The
antigenic determinants are degradation products of penicillins, particularly
penicilloic acid and products of alkaline hydrolysis bound to host protein. A
his-tory of a penicillin reaction is not reliable; about 5–8% of people claim
such a history, but only a small number of these will have an allergic reaction
when given penicillin. Less than 1% of persons who previously received
penicillin without incident will have an allergic reaction when given penicillin.
Because of the potential for anaphylaxis, however, penicillin should be
administered with cau-tion or a substitute drug given if the person has a
history of serious penicillin allergy. The incidence of allergic reactions in
young children is negligible.
Allergic reactions
include anaphylactic shock (very rare—0.05% of recipients); serum sickness-type
reactions (now rare—urticaria, fever, joint swelling, angioneurotic edema,
intense pruritus, and respiratory compromise occurring 7–12 days after
exposure); and a variety of skin rashes. Oral lesions, fever, interstitial
nephritis (an autoimmune reaction to a penicillin-protein complex),
eosino-philia, hemolytic anemia and other hematologic disturbances, and
vasculitis may also occur. Most patients allergic to penicillins can be treated
with alternative drugs. However, if necessary (eg, treat-ment of enterococcal
endocarditis or neurosyphilis in a patient with serious penicillin allergy),
desensitization can be accomplished with gradually increasing doses of penicillin.
In patients with renal
failure, penicillin in high doses can cause seizures. Nafcillin is associated
with neutropenia; oxacillin can cause hepatitis; and methicillin causes
interstitial nephritis (and is no longer used for this reason). Large doses of
penicillins given orally may lead to gastrointestinal upset, particularly
nausea, vomiting, and diarrhea. Ampicillin has been associated with
pseudomembranous colitis. Secondary infections such as vaginal candidiasis may
occur. Ampicillin and amoxicillin can cause skin rashes that are not allergic
in nature. These rashes frequently occur when aminopenicillins are
inappropriately prescribed for a viral illness.
Related Topics