Chapter: Basic & Clinical Pharmacology : Adrenoceptor Agonists & Sympathomimetic Drugs
Cardiovascular System - Organ System Effects of Sympathomimetic Drugs
ORGAN SYSTEM EFFECTS OF SYMPATHOMIMETIC DRUGS
Cardiovascular System
General
outlines of the cellular actions of sympathomimetics are presented in Tables
6–3 and 9–3. Sympathomimetics have promi-nent cardiovascular effects because of
widespread distribution of α and β adrenoceptors in the heart, blood vessels, and
neural and hormonal systems involved in blood pressure regulation. The net
effect of a given sympathomimetic in the intact organism depends
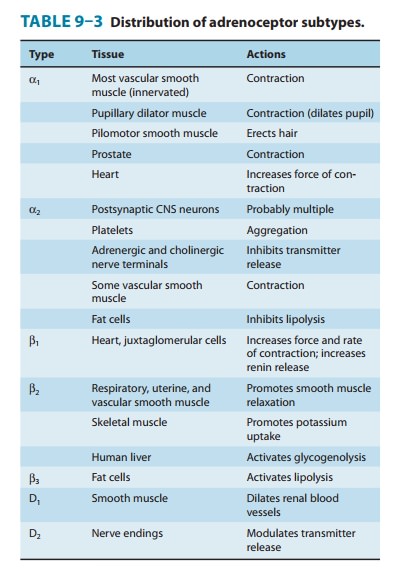
not
only on its relative selectivity for α or β adrenoceptors and its pharmacologic action at
those receptors; any effect these agents have on blood pressure is counteracted
by compensatory barore-flex mechanisms aimed at restoring homeostasis.
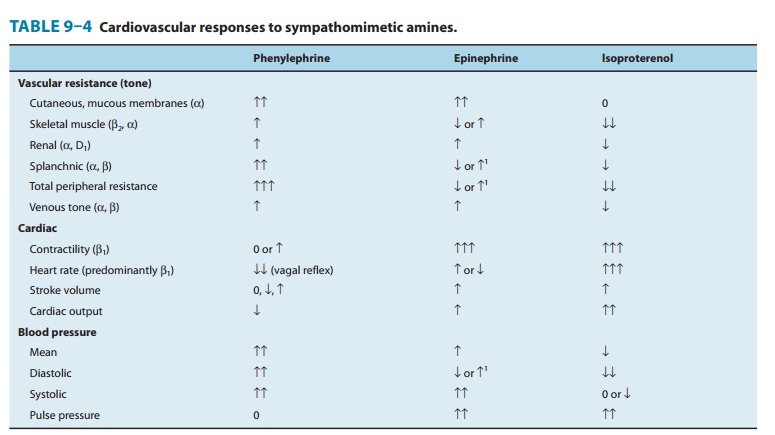
The effects of sympathomimetic drugs on blood pressure can be explained on the basis of their effects on heart rate, myocardial function, peripheral vascular resistance, and venous return (see Figure 6–7 and Table 9–4). The endogenous catecholamines, nor-epinephrine and epinephrine, have complex cardiovascular effects because they activate both α and β receptors. It is easier to under-stand these actions by first describing the cardiovascular effect of sympathomimetics that are selective for a given adrenoreceptor.
A. Effects of Alpha1-Receptor
Activation
Alpha1
receptors are widely expressed in vascular beds, and their activation leads to
arterial and venous vasoconstriction. Their direct effect on cardiac function
is of relatively less importance. A relatively pure α agonist such as phenylephrine increases
periph-eral arterial resistance and decreases venous capacitance. The enhanced
arterial resistance usually leads to a dose-dependent rise in blood pressure
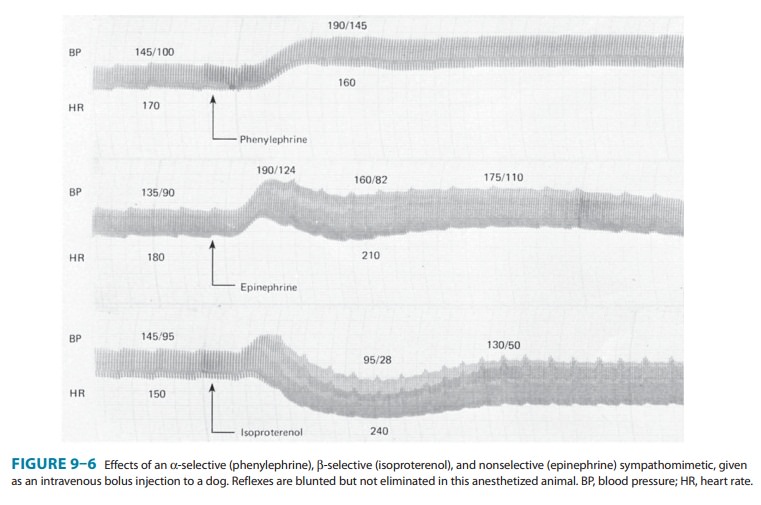
(Figure 9–6). In the presence of normal cardio-vascular reflexes, the rise in
blood pressure elicits a baroreceptor-mediated increase in vagal tone with
slowing of the heart rate, which may be quite marked (Figure 9–7). However,
cardiac out-put may not diminish in proportion to this reduction in rate, since
increased venous return may increase stroke volume. Furthermore, direct α-adrenoceptor
stimulation of the heart may have a modest positive inotropic action. The
magnitude of the restraining effect of the baroreflex is quite dramatic. If
baroreflex function isremoved by pretreatment with the ganglionic blocker
trimethaphan, the pressor effect of phenylephrine is increased approximately
tenfold, and bradycardia is no longer observed (Figure 9–7), con-firming that
the decrease in heart rate associated with the increase in blood pressure induced
by phenylephrine was reflex in nature rather than a direct effect of α1-receptor activation.
Patients
who have an impairment of autonomic function (due to pure autonomic failure as
in the case study or to more common conditions such as diabetic autonomic
neuropathy) exhibit this extreme hypersensitivity to most pressor and depressor
stimuli, including medications. This is to a large extent due to failure of
baroreflex buffering. Such patients may have exaggerated increases in heart
rate or blood pressure when taking sympathomimetics with β- and α-adrenergic activity,
respectively. This, however, can be usedas an advantage in their treatment. The
α
agonist midodrine is com-monly used to ameliorate orthostatic hypotension in
these patients.
There
are major differences in receptor types predominantly expressed in the various
vascular beds (Table 9–4). The skin vessels have predominantly α receptors and
constrict in response to epi-nephrine and norepinephrine, as do the splanchnic
vessels. Vessels in skeletal muscle may constrict or dilate depending on
whether α
or β
receptors are activated. The blood vessels of the nasal mucosa express α receptors, and local
vasoconstriction induced by sym-pathomimetics explains their decongestant
action (see Therapeutic Uses of Sympathomimetic Drugs).
B. Effects of Alpha2-Receptor Activation
Alpha2
adrenoceptors are present in the vasculature, and their acti-vation leads to
vasoconstriction. This effect, however, is observed only when α2 agonists are given
locally, by rapid intravenous injec-tion or in very high oral doses. When given
systemically, these vascular effects are obscured by the central effects of α2 receptors, which lead
to inhibition of sympathetic tone and blood pressure. Hence, α2 agonists are used as sympatholytics in the treatment of
hypertension . In patients with pure autonomic failure, characterized by neural
degeneration of postganglionic noradrenergic fibers, clonidine may increase
blood pressure because the central sympatholytic effects of clonidine become
irrelevant, whereas the peripheral vasoconstriction remains intact.
C. Effects of Beta-Receptor Activation
The
blood pressure response to a β-adrenoceptor agonist depends on its
contrasting effects on the heart and the vasculature. Stimulation of β receptors in the
heart increases cardiac output by increasing contractility and by direct
activation of the sinus node to increase heart rate. Beta agonists also
decrease peripheral resis-tance by activating β2 receptors, leading to vasodilation in certain
vascular beds (Table 9–4). Isoproterenol is a nonselective β agonist; it activates
both β1 and β2 receptors. The net effect is to maintain or slightly increase
systolic pressure and to lower diastolic pressure, so that mean blood pressure
is decreased (Figure 9–6).
Direct effects on the heart are determined largely by β1 recep-tors, although β2 and to a lesser extent α receptors are also involved, especially in heart failure. Beta-receptor activation results in increased calcium influx in cardiac cells. This has both electrical and mechanical consequences. Pacemaker activity— both normal (sinoatrial node) and abnormal (eg, Purkinje fibers)—is increased (positive chronotropic effect). Conduction velocity in the atrioventricular node is increased (positive dromo-tropic effect), and the refractory period is decreased. Intrinsiccontractility is increased (positive inotropic effect), and relax-ation is accelerated. As a result, the twitch response of isolated cardiac muscle is increased in tension but abbreviated in duration. In the intact heart, intraventricular pressure rises and falls more rapidly, and ejection time is decreased. These direct effects are eas-ily demonstrated in the absence of reflexes evoked by changes in blood pressure, eg, in isolated myocardial preparations and in patients with ganglionic blockade. In the presence of normal reflex activity, the direct effects on heart rate may be dominated by a reflex response to blood pressure changes. Physiologic stimulation of the heart by catecholamines tends to increase coronary blood flow. Expression of β3 adrenoreceptors has been detected in the human heart and may be upregulated in disease states, but its relevance to human disease is unclear.
D. Effects of Dopamine-Receptor Activation
Intravenous
administration of dopamine promotes vasodilation of renal, splanchnic,
coronary, cerebral, and perhaps other resistance vessels, via activation of D1
receptors. Activation of the D1 recep-tors in the renal vasculature
may also induce natriuresis. The renal effects of dopamine have been used
clinically to improve perfusion to the kidney in situations of oliguria
(abnormally low urinary output). The activation of presynaptic D2
receptors suppressesnorepinephrine release, but it is unclear if this
contributes to car-diovascular effects of dopamine. In addition, dopamine
activates β1receptors in the heart. At low doses, peripheral resistance
maydecrease. At higher rates of infusion, dopamine activates vascularreceptors,
leading to vasoconstriction, including in the renal vascular bed. Consequently,
high rates of infusion of dopamine may mimic the actions of epinephrine.
Related Topics