Chapter: Medical Physiology: Membrane Physiology, Nerve, and Muscle : Membrane Potentials and Action Potentials
Basic Physics of Membrane Potentials
Basic Physics of Membrane Potentials
Membrane Potentials Caused by Diffusion
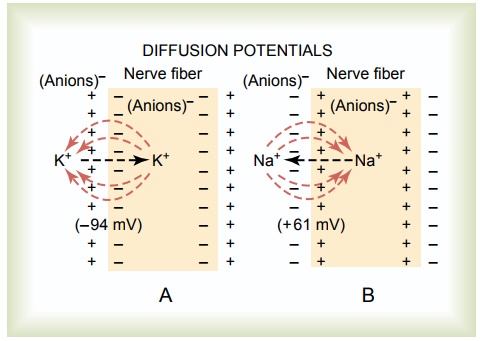
“Diffusion Potential” Caused by an Ion Concentration Difference on the Two Sides of the Membrane. In Figure 5–1A,the potassium concentration is greatinsidea nervefiber membrane but very low outside the membrane. Let us assume that the membrane in this instance is permeable to the potassium ions but not to any other ions. Because of the large potassium concentration gradient from inside toward outside, there is a strong tendency for extra numbers of potassium ions to diffuse outward through the membrane. As they do so, they carry positive electrical charges to the outside, thus creating electropositivity outside the membrane and electronegativity inside because of negative anions that remain behind and do not diffuse outward with the potassium. Within a millisecond or so, the potential difference between the inside and outside, called the diffusionpotential, becomes great enough to block further net potassium diffusion to theexterior, despite the high potassium ion concentration gradient. In the normal mammalian nerve fiber, the potential difference required is about 94 millivolts,with negativity inside the fiber membrane.
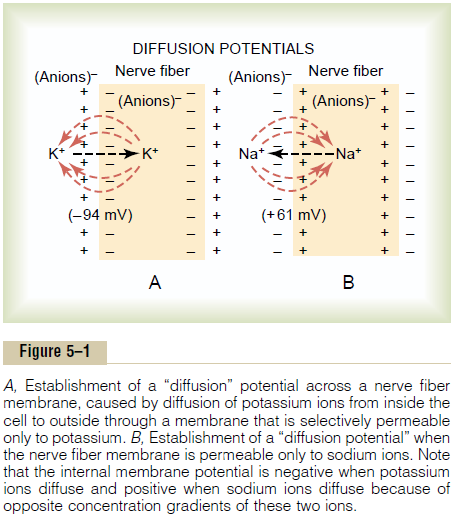
Figure 5–1B shows the same phenomenon as in Figure 5–1A, but this time with high concentration of sodium ions outside the membrane and low sodium inside. These ions are also positively charged. This time, the membrane is highlypermeable to the sodium ions but impermeable to all other ions. Diffusion of the positively charged sodium ions to the inside creates a membrane potential of opposite polarity to that in Figure 5–1A, with negativity outside and positiv-ity inside. Again, the membrane potential rises high enough within milliseconds to block further net diffusion of sodium ions to the inside; however, this time, in the mammalian nerve fiber, the potential is about 61 millivolts positive insidethe fiber.
Thus, in both parts of Figure 5–1, we see that a concentration difference of ions across a selectively permeable membrane can, under appropriate condi-tions, create a membrane potential. In later sections, we show that many of the rapid changes in membrane potentials observed during nerve and muscle impulse transmission result from the occurrence of such rapidly changing diffusion potentials.
Relation of the Diffusion Potential to the Concentration Differ-ence—The Nernst Potential. The diffusion potential levelacross a membrane that exactly opposes the net diffu-sion of a particular ion through the membrane is called the Nernst potential for that ion. The magnitude of this Nernst potential is determined by the ratio of the concentra-tions of that specific ion on the two sides of the mem-brane. The greater this ratio, the greater the tendency for the ion to diffuse in one direction, and therefore the greater the Nernst potential required to prevent additional net diffusion. The following equation, called theNernst equation, can be used to calculate the Nernst potential for any univalent ion at normal body temperature of 98.6°F (37°C):
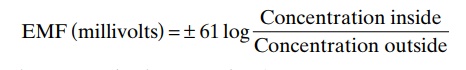
where EMF is electromotive force.
When using this formula, it is usually assumed that the potential in the extracellular fluid outside the membrane remains at zero potential, and the Nernst potential is the potential inside the membrane. Also, the sign of the potential is positive (+) if the ion dif-fusing from inside to outside is a negative ion, and it is negative (–) if the ion is positive. Thus, when the con-centration of positive potassium ions on the inside is 10 times that on the outside, the log of 10 is 1, so that the Nernst potential calculates to be –61 millivolts inside the membrane.
Calculation of the Diffusion Potential When the Membrane Is Permeable to Several Different Ions
When a membrane is permeable to several different ions, the diffusion potential that develops depends on three factors: (1) the polarity of the electrical charge of each ion, (2) the permeability of the membrane (P) to each ion, and (3) the concentrations (C) of the respective ions on the inside (i) and outside (o) of the membrane. Thus, the following formula, called the Goldman equation, or the Goldman-Hodgkin-Katz equation, gives the calculated membrane potential onthe inside of the membrane when two univalent posi-tive ions, sodium (Na+) and potassium (K+), and one univalent negative ion, chloride (Cl–), are involved.
EMF ( millivolts)
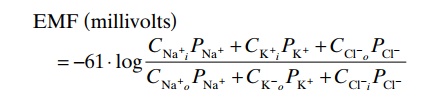
Let us study the importance and the meaning of this equation. First, sodium, potassium, and chloride ions are the most important ions involved in the develop-ment of membrane potentials in nerve and muscle fibers, as well as in the neuronal cells in the nervous system. The concentration gradient of each of these ions across the membrane helps determine the voltage of the membrane potential.
Second, the degree of importance of each of the ions in determining the voltage is proportional to the mem-brane permeability for that particular ion. That is, if the membrane has zero permeability to both potassium and chloride ions, the membrane potential becomes entirely dominated by the concentration gradient of sodium ions alone, and the resulting potential will be equal to the Nernst potential for sodium. The same holds for each of the other two ions if the membrane should become selectively permeable for either one of them alone.
Third, a positive ion concentration gradient from inside the membrane to the outside causes electroneg-ativity inside the membrane. The reason for this is that excess positive ions diffuse to the outside when their concentration is higher inside than outside. This carries positive charges to the outside but leaves the nondif-fusible negative anions on the inside, thus creating electronegativity on the inside. The opposite effect occurs when there is a gradient for a negative ion. That is, a chloride ion gradient from the outside to the inside causes negativity inside the cell because excess nega-tively charged chloride ions diffuse to the inside, while leaving the nondiffusible positive ions on the outside.
Fourth, as explained later, the permeability of the sodium and potassium channels undergoes rapid changes during transmission of a nerve impulse, whereas the permeability of the chloride channels does not change greatly during this process. Therefore, rapid changes in sodium and potassium permeability are primarily responsible for signal transmission in nerves.
Related Topics