Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Principles of Laboratory Diagnosis of Infectious Diseases
Antibody Detection (Serology)
Antibody Detection (Serology)
During infection — whether viral, bacterial, fungal, or parasitic — the host usually responds with the formation of antibodies, which can be detected by modification of any of the methods used for antigen detection. The formation of antibodies and their time course depends on the antigenic stimulation provided by the infection. The precise patterns vary depending on the antigens used, classes of antibody detected, and method. An example of temporal patterns of development and increase and decline in specific antiviral antibodies measured by different tests is illustrated in Figure 15 – 13. These responses can be used to detect evi-dence of recent or past infection. The test methods do not inherently indicate immunoglobu-lin class but can be modified to do so, usually by pretreatment of the serum to remove IgG to differentiate the IgM and IgG responses. Several basic principles must be emphasized:
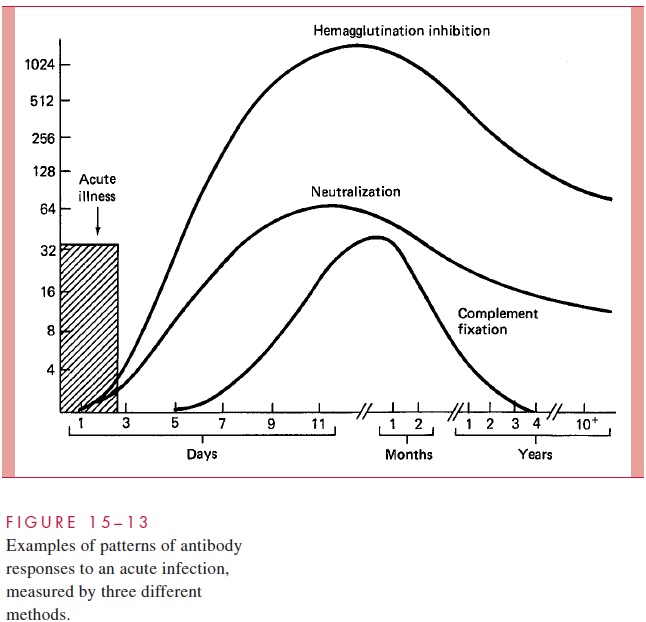
1. In an acute infection, the antibodies usually appear early in the illness, and then rise sharply over the next 10 to 21 days. Thus, a serum sample collected shortly after the onset of illness (acute serum) and another collected 2 to 3 weeks later (convalescent serum) can be compared quantitatively for changes in specific antibody content.
2. Antibodies can be quantitated by several means. The most common method is to di-lute the serum serially in appropriate media and determine the maximal dilution that will still yield detectable antibody in the test system (eg, serum dilutions of 1:4, 1:8, and 1:16). The highest dilution that retains specific activity is called the antibody titer.
3. The interpretation of significant antibody responses (evidence of specific, recent in-fection) is most reliable when definite evidence of seroconversion is demonstrated; that is, detectable specific antibody is absent from the acute serum (or preillness serum, if available) but present in the convalescent serum. Alternatively, a fourfold or greater increase in antibody titer supports a diagnosis of recent infection; for example, an acute serum titer of 1:4 or less and a convalescent serum titer of 1:16 or greater would be considered significant.
4. In instances in which the average antibody titers of a population to a specific agent are known, a single convalescent antibody titer significantly greater than the expected mean may be used as supportive or presumptive evidence of recent infection. How-ever, this finding is considerably less valuable than those obtained by comparing re-sponses of acute and convalescent serum samples. An alternative and somewhat more complex method of serodiagnosis is to determine which major immunoglobulin sub-class constitutes the major proportion of the specific antibodies. In primary infections, the IgM-specific response is often dominant during the first days or weeks after onset but is replaced progressively by IgG-specific antibodies; thus, by 1 to 6 months after infection, the predominant antibodies belong to the IgG subclass. Consequently, serum containing a high titer of antibodies of the IgM subclass would suggest a re-cent, primary infection.
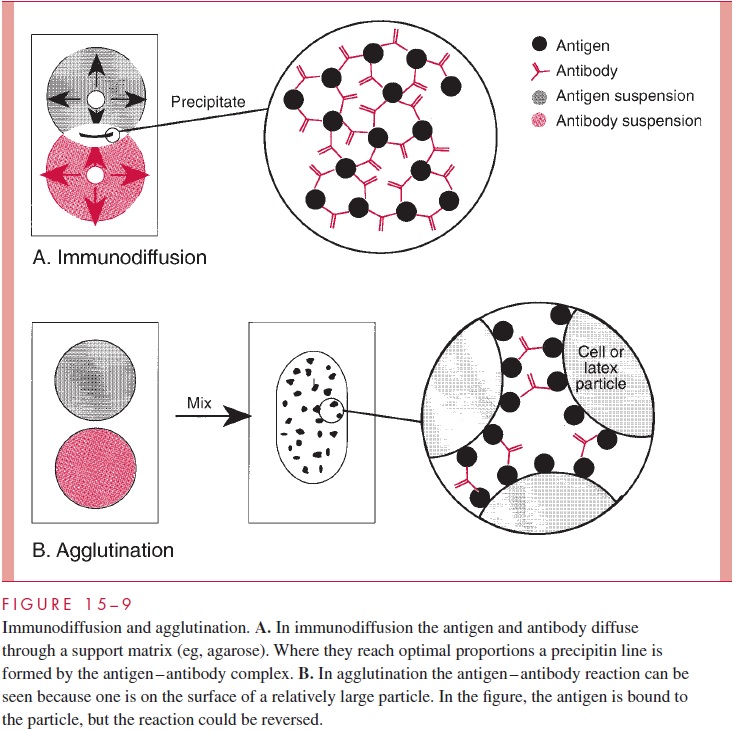
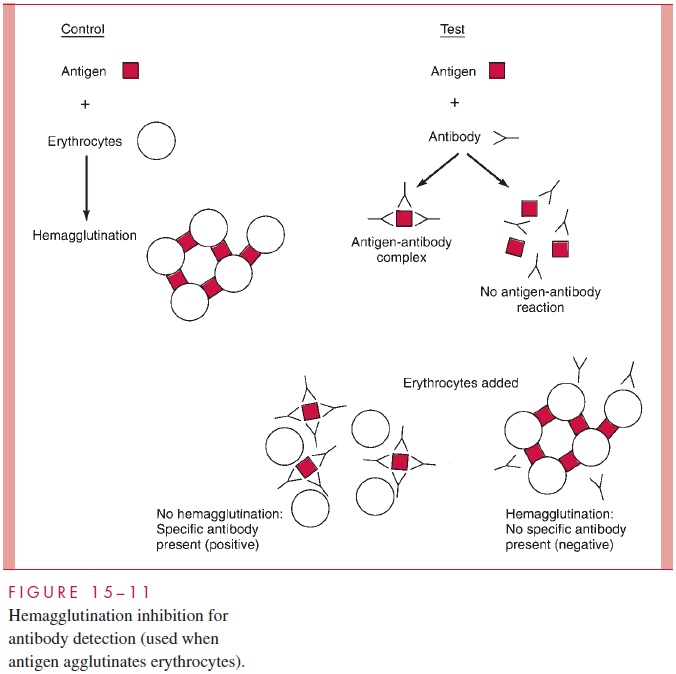
The immunologic methods used to identify bacterial or viral antigens are applied to serologic diagnosis by simply reversing the detection system: that is, using a known rather than an unknown antigen to detect the presence of an antibody. The methods of serologic diagnosis to be used are selected on the basis of their convenience and applicability to the antigen in question. As shown in Figure 15 – 13, the temporal relationships of antibody response to infection vary according to the method used. Of the methods for measuring antigen – antibody interaction discussed previously, those now used most frequently for serologic diagnosis are agglutination, RIA, and EIA (see Figs 15 – 9 and 15 – 11).
Western Blot
The Western blot immunoassay is another technique that is now commonly employed to detect and confirm the specificity of antibodies to a variety of epitopes. Its greatest use has been in the diagnosis of HIV infections , in which virions are elec-trophoresed in a polyacrylamide gel to separate the protein and glycoprotein components and then transferred onto nitrocellulose. This is then incubated with patient serum, and antibody to the different viral components is detected by using an antihuman globulin IgG antibody conjugated with an enzyme label.
Related Topics