Chapter: 12th Chemistry : UNIT 13 : Organic Nitrogen Compounds
Amines - classification, Structure, Nomenclature
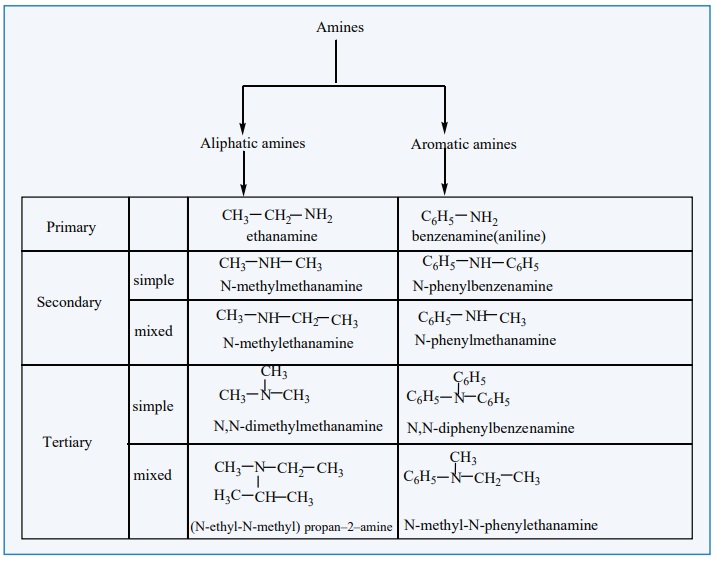
Amines - classification
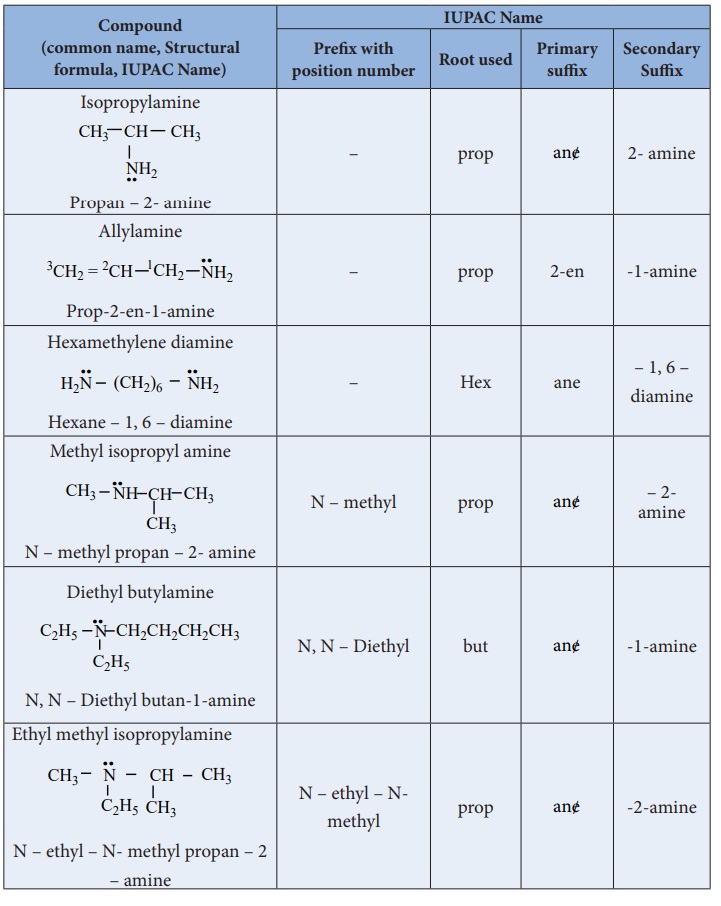
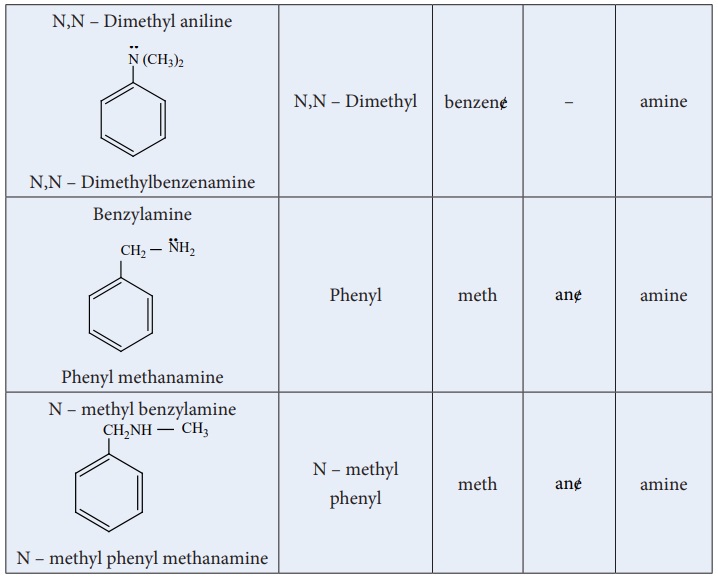
Nomenclature
a) Common system:
In common system, an aliphatic amine is named by prefixing alkyl group to amine. The prefixes di-,tri-, and tetra-, are used to describe two, three(or) four same substituent’s.
b) IUPAC System:
Evaluate yourself
Draw the structure of the following compounds
i. Neopentylamine
ii. Tert – butylamine
iii. α- amino propionaldehyde
iv. Tribenzylamine
v. N – ethyl – N – methylhexan – 3- amine
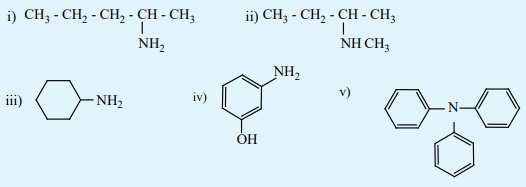
8) Give the correct IUPAC names for the following amines
Structure of amines
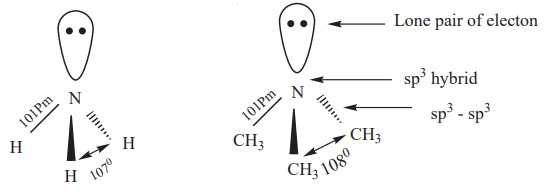
Like, ammonia, nitrogen atom of amines is trivalent and carries a lone pair of electron and sp3 hybridised, out of the four sp3 hybridised orbitals of nitrogen, three sp3 orbitals overlap with orbitals of hydrogen (or) alkyl groups of carbon, the fourth sp3 orbital contains a lone pair of electron. Hence, amines posses pyramidal geometry. Due to presence of lone pair of electron C - N- H (or) C- N- C bond angle is less than the normal tetrahedral bond angle 109.50. For example, the C- N- C bond angle of trimethylamine is 1080 which is lower than tetrahedral angle and higher than the H- N- H bond angle of 107˚ . This increase is due to the repulsion between the bulky methyl groups.
Related Topics