Chapter: Biology of Disease: Disorders of the Gastrointestinal Tract, Pancreas, Liver and Gall Bladder
Absorption of the Products of Digestion
ABSORPTION OF THE PRODUCTS OF
DIGESTION
The large surface area of the small intestine allows the rapid
absorption of the products of digestion. The enzymes concerned with the final
stages of diges-tion of a number of nutrients are located in the brush border
of the entero-cytes as described or even within their cytoplasm. This ensures
that the final products of digestion are produced near or within the
absorp-tive surface of the GIT. Enterocytes are joined together by tight junctions
that ensure material cannot leak from the lumen. Absorption by enterocytes is
largely active and selective and they have a high metabolic rate because the
transport of materials across their membranes requires considerable amounts of
metabolic energy. A membrane-bound Na+/K+-ATPase uses a
major pro-portion of this energy to catalyze the hydrolysis of ATP in the
presence of Na+ and K+. The free energy from the
hydrolysis is used to expel three Na+ from the cell and to pump two
K+ into the cell. This may be summarized as:
3Na+IN
+ 2K+OUT + ATP
+ H2O -> 3Na+OUT + 2K+IN
+ ADP + Pi
Since both Na+ and K+ are being
transported against their electrochemical gradients both movements are an
active transport. Also, since more positive charges are being pumped out of the
cell than are entering it, the effect con-tributes to the potential difference
across the membrane, called the resting membrane potential, of about –60 mV,
the inside of the cell being negative with respect to the outside. The membrane
potential of the apical membrane of the enterocyte can be harnessed to
facilitate the uptake of a variety of nutri-ents from the lumen of the GIT.
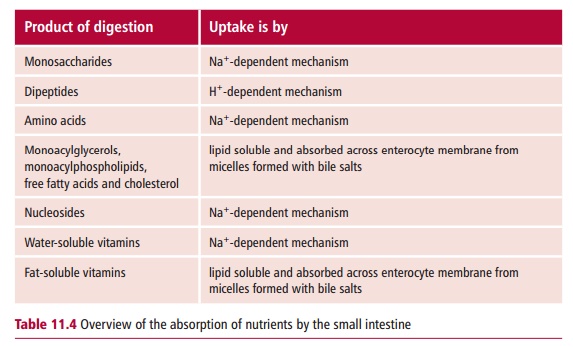
Both amino acids and monosaccharides are transported across the enterocyte
luminal membrane in a Na+-dependent fashion. A variety of different
membrane transporter proteins are responsible for the absorption of specific
sugars and different groups of amino acids.
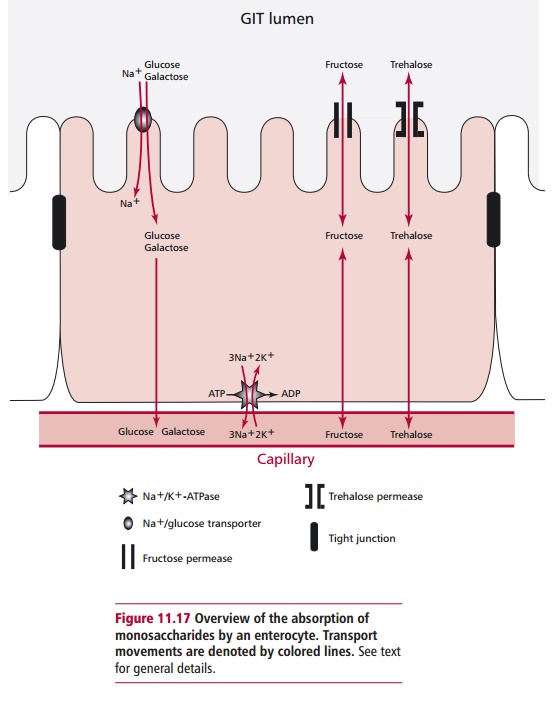
Glucose and galactose are transported across the enterocyte
luminal mem-brane in an active, Na+-dependent fashion by the same
transporter. One mol-ecule of these sugars can only move through the
transporter into the cell if Na+ ions move in at the same time (Figure 11.17). The concentrations of the
sugars can build up within the cytoplasm, such that they are able to leave the
cell through the basolateral membrane by facilitated diffusion. Other
mono-saccharides, for example fructose and trehalose, are absorbed only by
facili-tated diffusion and are absorbed to a much lesser extent.
The major initial products of protein digestion are small
peptides and these are absorbed by enterocytes of the jejunum at their luminal
surfaces by pep-tide transporter protein, called the PepT1 (Figure 11.18). This occurs in a H+-dependent
fashion that resembles the uptake of glucose and galactose. Within the
cytoplasm, the peptides are hydrolyzed to amino acids, ensuring a con-tinuous
sink is present to facilitate peptide uptake by the cells. The exit of the
amino acids from the cells on the basolateral side also occurs down their
con-centration gradients. However, as peptides are moved further along the GIT,
they are hydrolyzed by peptidases to free amino acids and their absorption
occurs in the ileum using a number of Na+-dependent transporters (Figure11.18), which have specificities
for different amino acid side chains. Peptides
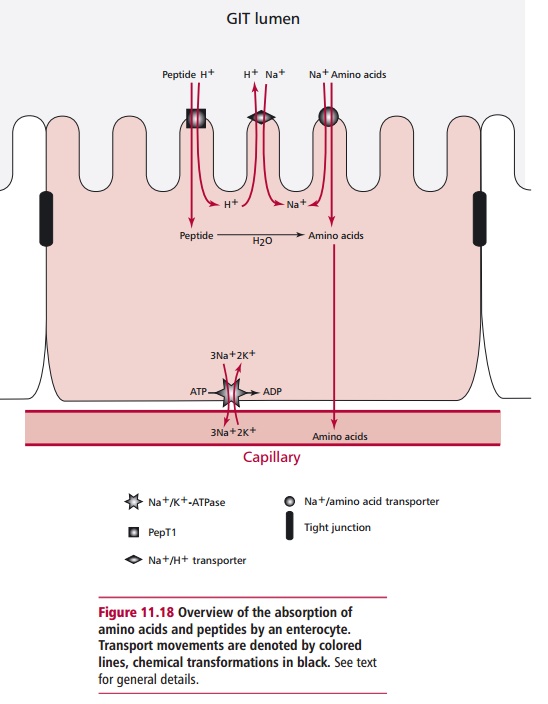
can also be absorbed by a paracellular route where they pass
between entero-cytes, rather than being absorbed across the luminal surface.
Relatively large peptides can be absorbed by this method and may initiate an
allergic reaction leading to food allergies.
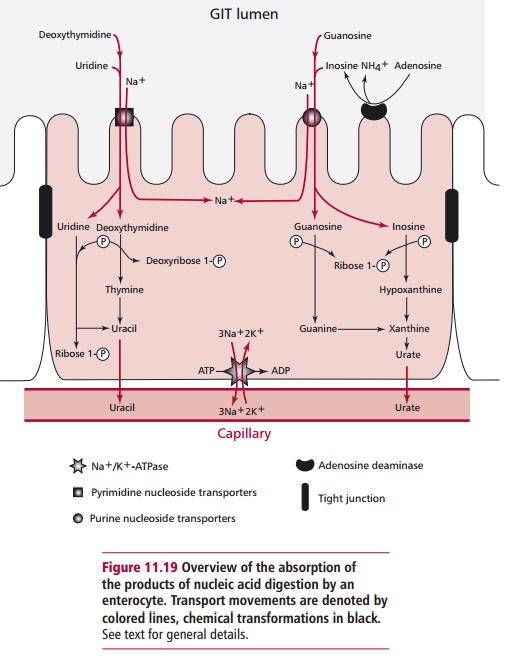
Digestion of RNA produces nucleotides that are further degraded
to nucle-osides at the brush border and which, again, are absorbed in a Na+-dependent
manner. Catabolism within the cytoplasm converts the nucleotides to ribose
phosphate and bases. Eventually the purine bases are converted to urate and the
pyrimidines to uracil as shown in Figure
11.19.
Fatty acids, monoacylglycerols, monoacylphospholipids and
cholesterol are absorbed as mixed micelles by the brush border of the
enterocytes (Figure11.20). The
triacylglycerols and phospholipids are reformed within the ente-rocyte
cytoplasm and packaged into large lipoprotein complexes called chy-lomicrons that are transported from
the GIT in lacteals of thelymphatic system. This ensures the lipids bypass the
liver and are delivered to the blood through the thoracic duct.
Water-soluble vitamins are taken up by enterocytes by a variety
of mecha-nisms. Vitamins B1 (thiamin) and B2 (riboflavin)
are absorbed in the upper portion of the small intestine. Thiamin is actively
transferred to the portal system. Specific transporter proteins actively
accumulate niacin (nicotinic acid and nicotinamide), folic acid and biotin
(vitamin H) in Na+-dependent fashions. Pantothenic acid and the
vitamers of vitamin B6 are absorbed by diffusion. Vitamin C is
absorbed in the jejunum by a Na+-dependent mecha-nism, similar to
that described for glucose. The fat-soluble vitamins, A, D, E and K, are
absorbed within the mixed micelles of fatty acids, monoacylglyc-
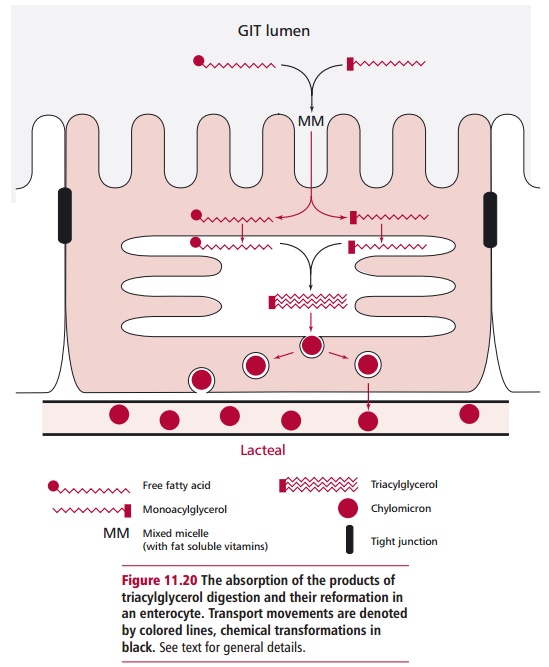
erols, monoacylphospholipids and cholesterol described above (Figure 11.20) and leave the enterocyte
in the chylomicrons. For these reasons, a deficiency in dietary lipids means
that the absorption of fat-soluble vitamins is greatly reduced.
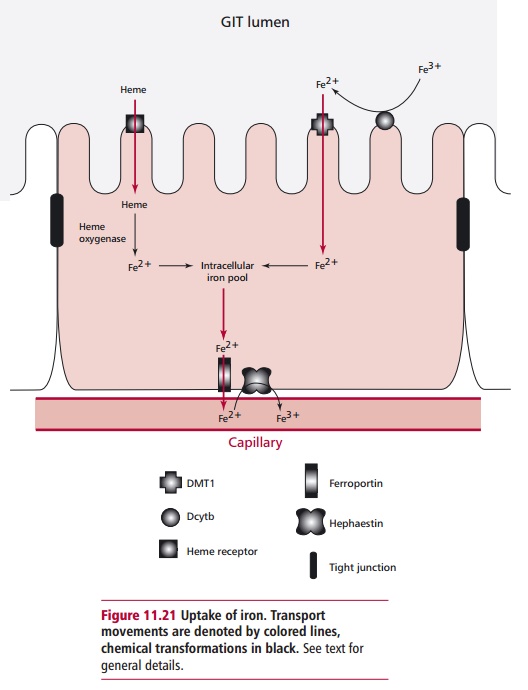
Many minerals are absorbed in an energy dependent fashion along the length of the GIT, although Ca2+ and iron are mainly absorbed in the duodenum. Dietary iron occurs in nonheme and heme forms and their
absorption by duodenal enterocytes is by different mechanisms. Dietary nonheme
iron occurs in ferrous (Fe(II)2+) and ferric (Fe(III)3+)
forms. Ferrous iron is solu-ble up to a pH of about 7 but the predominant form,
ferric iron, is sparingly soluble above pH 3 and is not available for
absorption and must be reduced before it can be transported across the
intestinal epithelium. A ferrireductase called Dcytb bound to the brush border
of enterocytes reduces ferric iron to the ferrous form. Ferrous iron is then
transported into the cell by a H+ coupled mechanism as illustrated
in Figure 11.21. The transporter,
called the divalent metal transporter 1 (DMT1), is also able to effect the
absorption of a number of other divalent metal ions, such as those of cadmium,
cobalt copper, lead, nickel and zinc. Heme iron is absorbed into the enterocyte
by a heme receptor and, once internalized, its ferrous iron is released into
the intracellular pool by heme oxygenase activity (Figure 11.21). Ferrous iron is exported from the enterocyte across
the basal membrane by a membrane protein called ferro-portin1 or Ireg1. It is
then oxidized by hephaestin, a transmembrane copper dependent ferroxidase,
which is necessary for effective iron transport. The ferric iron is bound by
transferrin in the plasma and can be stored in erythro-cytes in ferritin
molecules .
Calcium is absorbed in the upper part of the small intestine in
an ionic form. This absorption requires the active metabolite of vitamin D,
1,25-dihydroxy-vitamin D3, and is inhibited by substances that form
insoluble calcium salts, such as phosphate and oxalate. The uptake of Na+
has been mentioned already in relation to the active uptake of several
nutrients and many anions, hydro-gen carbonate, chloride and iodide, can
passively follow it into enterocytes. Phosphate is actively accumulated by
enterocytes.
Amino acids, monosaccharides, urate and uracil, B vitamins,
vitamin C and minerals all leave the enterocytes through their basolateral
membranes, enter the hepatic portal vein and are delivered to the liver.
Following their absorption, many minerals are bound by intracellular proteins
before being expelled through the basolateral membrane into the bloodstream
where they
are bound by transport proteins, such as transferrin for iron (Figure 13.4) and ceruloplasmin for
copper. Table 11.4 summarizes the
mechanisms of absorp-tion of the major nutrients.
Approximately 9 dm3 of fluid pass through the GIT
each day. Reabsorption of water from the GIT is essential to prevent
dehydration. Most, about 95%, is absorbed by the small intestine, 4% by the
large intestine and only 1% is lost from the GIT.
Related Topics