Definition, Main assumptions, Limitations | Theories of coordination compound | Chemistry - Valence Bond Theory | 12th Chemistry : UNIT 5 : Coordination Chemistry
Chapter: 12th Chemistry : UNIT 5 : Coordination Chemistry
Valence Bond Theory
Valence Bond Theory
According to this theory, the bond formed between the central metal atom and the ligand is due to the overlap of filled ligand orbitals containing a lone pair of electron with the vacant hybrid orbitals of the central metal atom.
Main assumptions of VBT:
1. The ligand → metal bond in a coordination complex is covalent in nature. It is formed by sharing of electrons (provided by the ligands) between the central metal atom and the ligand.
2. Each ligand should have at least one filled orbital containing a lone pair of electrons.
3. In order to accommodate the electron pairs donated by the ligands, the central metal ion present in a complex provides required number (coordination number) of vacant orbitals.
4. These vacant orbitals of central metal atom undergo hybridisation, the process of mixing of atomic orbitals of comparable energy to form equal number of new orbitals called hybridised orbitals with same energy.
5. The vacant hybridised orbitals of the central metal ion, linearly overlap with filled orbitals of the ligands to form coordinate covalent sigma bonds between the metal and the ligand.
6. The hybridised orbitals are directional and their orientation in space gives a definite geometry to the complex ion.
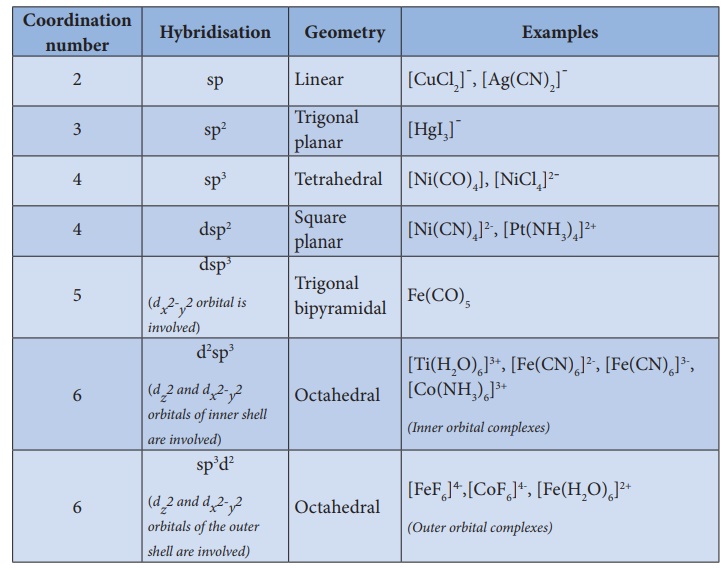
7. In the octahedral complexes, if the (n-1) d orbitals are involved in hybridisation, then they are called inner orbital complexes or low spin complexes or spin paired complexes. If the nd orbitals are involved in hybridisation, then such complexes are called outer orbital or high spin or spin free complexes. Here n represents the principle quantum number of the outermost shell.
8. The complexes containing a central metal atom with unpaired electron(s) are paramagnetic. If all the electrons are paired, then the complexes will be diamagnetic.
9. Ligands such as CO, CN-, en, and NH3 present in the complexes cause pairing of electrons present in the central metal atom. Such ligands are called strong field ligands.
10. Greater the overlapping between the ligand orbitals and the hybridised metal orbital, greater is the bond strength.
Let us illustrate the VBT by considering the following examples.
Illustration 1
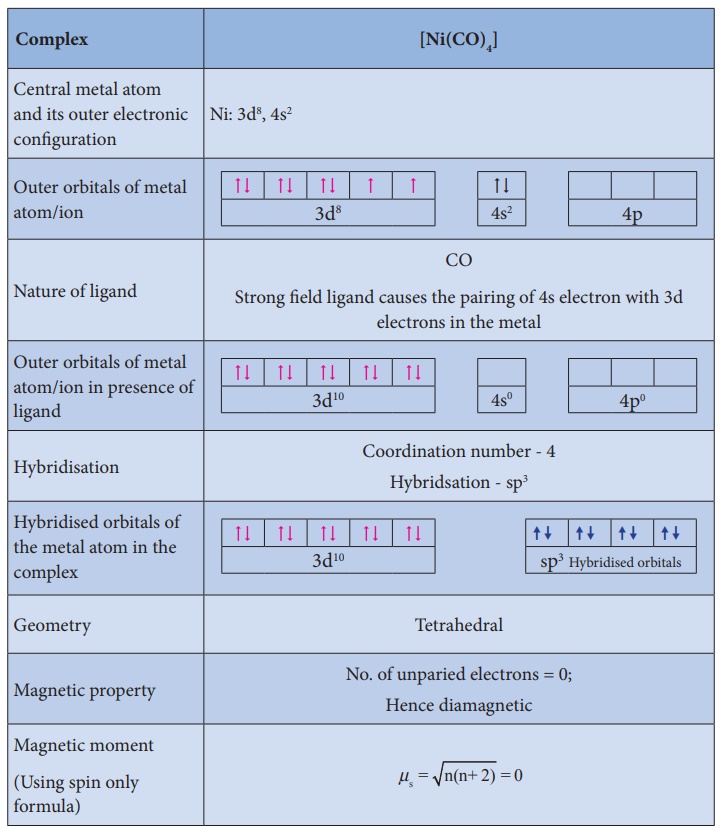
Illustration 2
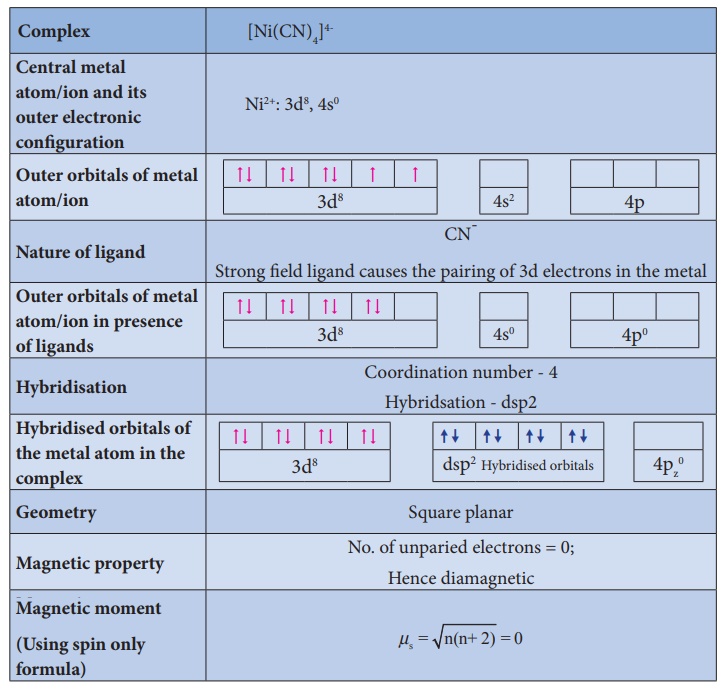
Illustration 3
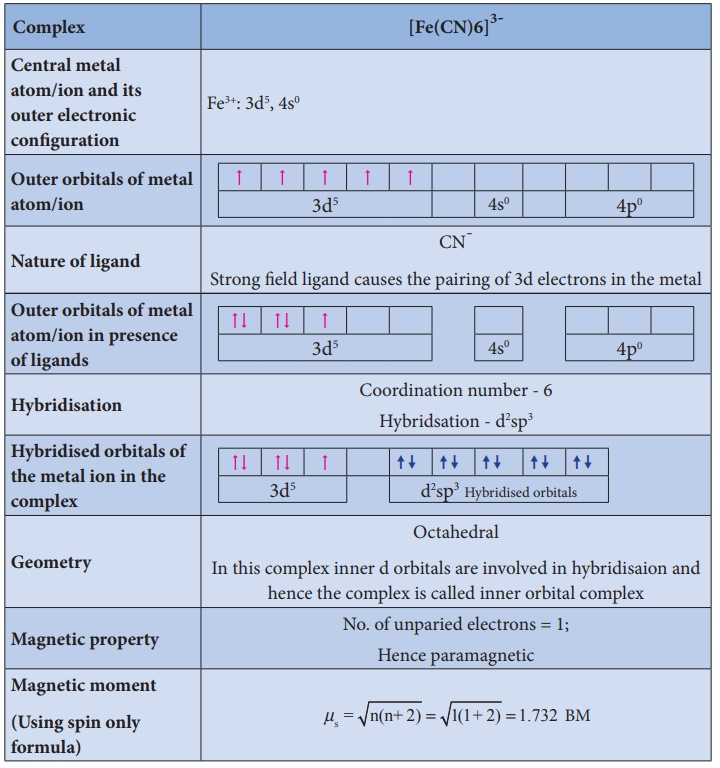
Illustration 4
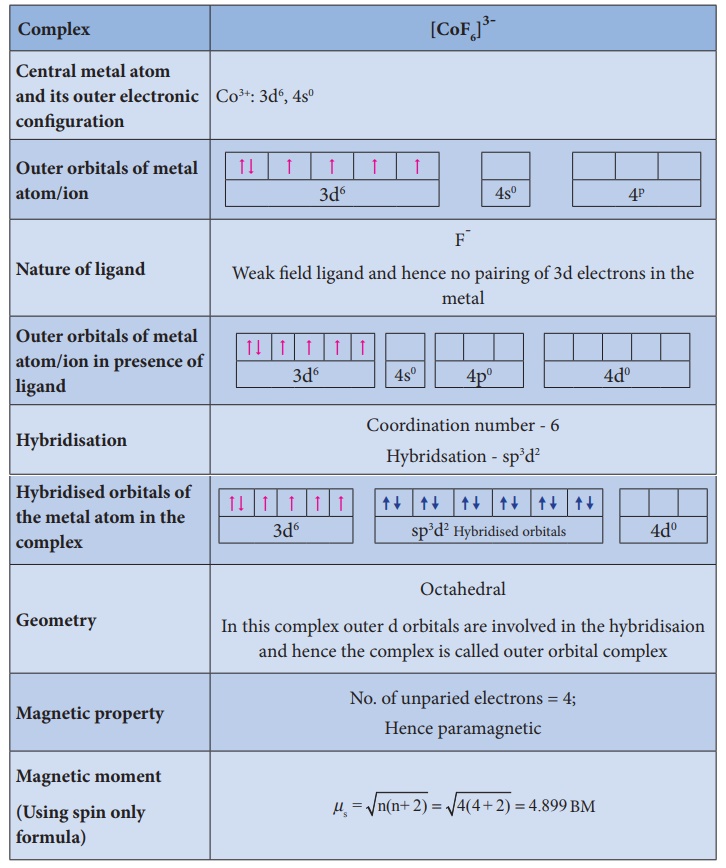
Limitations of VBT
Eventhough VBT explains many of the observed properties of complexes, it still has following limitations
1. It does not explain the colour of the complex
2. It considers only the spin only magnetic moments and does not consider the other components of magnetic moments.
3. It does not provide a quantitative explanation as to why certain complexes are inner orbital complexes and the others are outer orbital complexes for the same metal. For example, [Fe(CN)6]4- is diamagnetic (low spin) whereas [FeF6]4- is paramagnetic (high spin).
Related Topics