Chapter: Medical Immunology: AIDS and Other Acquired Immunodeficiency Diseases
Secondary Immunodeficiencies
SECONDARY IMMUNODEFICIENCIES
A. Immunodeficiency Associated with Malnutrition
Immunodeficiency secondary to malnutrition has been reported in association with generalized malnutrition or in association with vitamin, mineral, and trace element deficiencies. Severe protein-calorie malnutrition is primarily associated with a depression of cell-medi-ated immunity. Different groups have reported anergy, low T-lymphocyte counts, de-pressed lymphocyte reactivity to PHA, and depressed cytokine release in malnourished populations. In kwashiorkor, which is due to a combination of protein-calorie malnutrition and deficiency in trace elements and vitamins, the degree of immunodeficiency seems to be more profound. Affected children seem to have a delayed maturation of the B-cell sys-tem and often have low levels of mucosal IgA, without apparent clinical reflection. Efforts to study the humoral immune response to active immunization have yielded variable re-sults. The complement system and neutrophil functions have been reported as depressed, but the phagocytic impairment is mild and depressed complement levels seem to be pri-marily a result of consumption as a consequence of infections.
Several causes for the immune deficiency associated with malnutrition have been suggested, including general metabolic depression, thymic atrophy with low levels of thymic factors, depressed numbers of helper T lymphocytes (which could account for the variable compromise of humoral immunity), and impaired cytokine release. A practical consideration to bear in mind is that malnourished children should not be vaccinated with live, attenuated vaccines, which are generally contraindicated in immunodeficient patients.
B. Immunodeficiency Associated with Zinc Deficiency
The significance of zinc deficiency for the normal functioning of the immune system is un-derlined by observations performed in patients with acrodermatitis enteropathica, a rare congenital disease in which diarrhea and malabsorption (affecting zinc, among other nutri-ents) play a key pathogenic role. Affected patients often present with epidermolysis bullosa and generalized candidiasis, associated with combined immunodeficiency that can be cor-rected with zinc supplementation.
Secondary zinc deficiency are considerably more frequent and can develop as a con-sequence of low meat consumption, high-fiber diet, chronic diarrhea, chronic kidney in-sufficiency, anorexia nervosa and bulimia, alcoholism, diabetes, psoriasis, hemodialysis, parenteral alimentation, etc. The depletion caused by these conditions does not seem to be severe enough to cause symptomatic immunodeficiency, but it may be one of several fac-tors adversely affecting the immune system.
The basis for the depression of cell-mediated immunity in zinc deficiency is not fully known, but it has been proposed that zinc may be essential for the normal activity of cellu-lar protein kinases involved in signal transduction during lymphocyte activation.
C. Immunodeficiency Associated with Vitamin Deficiencies
Several vitamin deficiencies are associated with and are presumably the cause of abnor-malities of the immune response, particularly when associated with protein-calorie malnu-trition. The molecular mechanisms underlying these deficiencies have not been defined. Deficiencies of pyridoxine, folic acid, and vitamin A are usually associated with cellular immunodeficiency. Panthotenic acid deficiency is usually associated with a depression of the primary and secondary humoral immune responses. Vitamin E deficiency is associated with a combined immunodeficiency.
D. Immunodeficiency Associated with Renal Failure
Patients with renal failure have depressed cell-mediated immunity, as reflected in cuta-neous anergy, delayed skin graft rejection, lymphopenia, and poor T-lymphocyte responses to mitogenic stimulation. Humoral immunity can also be affected, particularly in patients with the nephrotic syndrome, who may lose significant amounts of IgG in their urine. Sev-eral factors seem to contribute to the depression of cell-mediated immunity in patients with renal failure:
1. Release of a soluble suppressor factor, as shown by experiments demonstrating that plasma or serum from uremic patients suppresses the mitogenic responses of normal lymphocytes in vitro. The responsible factors have a molecular weight less than 20,000, and it has been suggested that methylguanidine and “middle molecules” (molecular weight 1200) are responsible. These molecules can be isolated from uremic sera and have been shown to suppress in vitro mitogenic re-sponses of normal T lymphocytes.
2. In dialyzed patients there is a paradoxical activation of the immune system, which results in excessive and unregulated release and consumption of IL-2, re-sulting in decreased bioavailability of this cytokine.
3. Fc-mediated phagocytosis is impaired in patients with severe renal failure, perhaps secondary to increased levels of endogenous glucocorticoid levels. There is also evidence of a compromise of the capacity of monocytes to function as antigen-pre-senting cells. These abnormalities are reproducible when normal monocytes are incubated with uremic serum. Dialysis may accentuate the problem as a conse-quence of complement activation in the dialysis membranes, which causes a poorly understood downregulation of the expression of CAMs by phagocytic cells.
4. Patients with chronic renal failure secondary to autoimmune diseases are often treated with immunosuppressive drugs that further compromise the immune sys-tem.
E. Burn-Associated Immunodeficiency
Bacterial infections are a frequent and severe complication in burn patients, often leading to death. Several factors may contribute to the incidence of infections in burned patients, including the presence of open and infected wounds, a general metabolic disequilibrium, and a wide spectrum of immunological abnormalities.
Depressed neutrophil function is a major factor contributing to the lowered resistance to infection. Defective chemotaxis and reduced respiratory burst are the most prominent ab-normalities. Several factors may contribute to this depression:
1. Exaggerated complement activation (mostly by proteases released in injured tis-sues) causes the release of large concentrations of C5a, which may disturb proper chemotactic responses and cause massive activation of granulocytes. When the already activated granulocytes reach the infected tissues, they may no longer be responsive to additional stimulation.
2. Bacterial endotoxin, prostaglandins, and β-endorphins have been suggested as additional factors that adversely affect phagocytic cell functions. The involve-ment of prostaglandins has been supported by studies in experimental animals, in which administration of cyclooxygenase blockers normalizes phagocytic cell functions.
3. Another contributing factor seems to be the low opsonizing power of the burn blister fluid, which has very low levels of both complement and immunoglobu-lins.
Impairment of cell-mediated immunity is suggested by a prolongation of skin homo-graft survival and depressed delayed-hypersensitivity responses. Laboratory studies show have documented low responses to mitogenic stimuli and depressed mixed lymphocyte cul-ture reactions. A major functional abnormality of T lymphocytes isolated from burned pa-tients is their depressed release of IL-2 after mitogenic stimulation. This depression may be secondary to the release of immunosuppressive factors by the burned tissues, including a 10 kDa glycopeptide, a 1000 kDa lipid-protein complex, and PGE2, released by overactive monocytes, which causes an increase of intracellular cAMP in T cells, resulting in an inhi-bition of cell proliferation.
F. Iatrogenically Induced Immune Deficiencies
A wide range of therapeutic interventions has been show to cause functional depression of the immune system. At the top of the list is the administration of cytotoxic/immunosup-pressive drugs , but many other medical procedures have unexpected ef-fects on the immune system.
1. Neutropenia
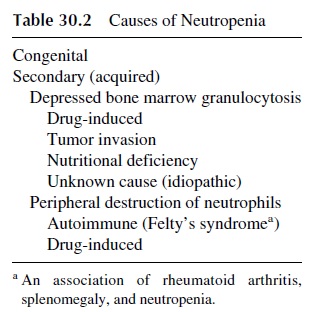
The reduction of the total number of neutrophils is the most frequent cause of infection due to defective phagocytosis. Although there are rare congenital forms of neutropenia of vari-able severity, most frequently neutropenia is secondary to a variety of causes (see Table 30.2). Administration of cytotoxic drugs is almost inevitable followed by neutropenia , but a variety of drugs of other groups may cause idiosyncratic neutropenia with variable frequency.
2. Postsurgery Immunodeficiency
Both surgery and general anesthesia are associated with transient depression of immune functions, affecting the mitogenic responses of PBL, cutaneous hypersensitivity, and anti-body synthesis. Multiple factors seem to contribute to the depression of the immune sys-tem (Fig. 30.1):
A transient severe lymphopenia can occur in the immediate postoperative period. Exaggerated release of PGE2, due to the posttraumatic activation of inflammatory cells, depresses T-lymphocyte and accessory cell functions.
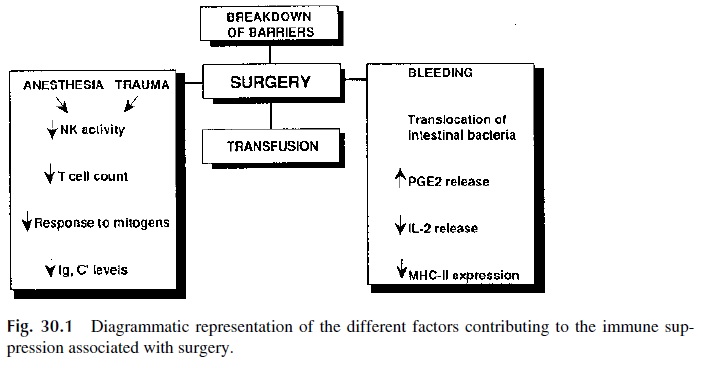
· Blood loss can be associated with a reduction of IL-2 release by activated T lympho-cytes and of MHC-II expression by accessory cells and with reduced B-lym-phocyte responses to antigenic stimulation.
· Transfusions have a poorly understood immunosuppressive effect.
· Anesthesia and administration of opiates (as painkillers) can lead to a depression of phagocytic cell functions and to a reduced activity of NK cells.
Complete normalization of immune function may take 10 days (for mitogenic re-sponses) to a month (for delayed hypersensitivity reactions and the humoral immune re-sponse).
It also needs to be kept in mind that postsurgical infection is facilitated by a variety of factors associated with surgery, which even in its simplest form is traumatic to the pa-tient. For example, the surgical incision disrupts the integrity of the skin, a very important barrier against infection. Special types of surgery, such as intestinal surgery, promote spreading of bacteria from a highly contaminated organ into surrounding tissues. The in-troduction of intravenous lines and catheters, often associated with surgical procedures, opens new routes for the penetration of opportunistic agents. Severe blood loss during surgery can cause massive entrance of intestinal bacteria into the portal circulation and, subsequently, into the systemic circulation (phenomenon known as bacterial transloca-tion). If one adds a depressed immune system to these factors, its is easy to understand theclinical significance of postsurgical infection.
3. Splenectomy
Splenectomy deserves special reference as a cause of immune depression. The removal of the spleen represents the loss of an important filtration organ, very important for the removal of circulating bacteria. In addition, the spleen plays a significant role in recruit-ing immunocytes in the initial phases of the immune responses. Splenectomized patients are weakly responsive to polysaccharides, and if we add this fact to the inability to re-move bacteria, particularly those with polysaccharide capsules, from circulation, it is easy to understand why splenectomized patients are prone to severe septicemia. The most commonly offending organisms include Streptococcus pneumoniae (50% of the cases),
Haemophilus influenzae, and Neisseria meningitidis—all of them pyogenic bacteriawith antiphagocytic polysaccharide capsules. Other organisms involved as frequent causes of infection in splenectomized patients include Staphylococcus aureus and group A Streptococcus. It has also been demonstrated that about one third of the cases of hu-man infection by Babesia, an intracellular sporozoan, have occurred in splenectomized patients.
Similar defects are noticed in patients with sickle cell anemia, who develop splenic atrophy as a consequence of repeated infections and fibrosis (autosplenectomy). Notewor-thy is the fact that patients with sickle cell anemia are particularly prone to develop salmonella bacteremia involving strains of Salmonella enteritidis, which do not dissemi-nate in the blood stream of normal individuals. This allows S. enteritidis to spread to organs other than the intestine, namely the bones (S. enteritidis is the most frequent cause of os-teomyelitis in patients with sickle cell anemia).
4. Thymectomy
Thymectomy is frequently done in neonates with congenital heart disease to ensure proper surgical access. It is generally believed that thymectomy after birth has few (if any) effects on the development of the immune system of humans, and there is no conclusive evidence suggesting otherwise.
G. Immunosuppression Associated with Drug Abuse
There is considerable interest in defining the effects of drug abuse on the immune system. Unfortunately, most data concerning the immunological effects of drugs of abuse are based on in vitro experiments or on studies carried out with laboratory animals, which may or may not reflect the in vivo effects of these compounds in humans.
1. Alcohol
Chronic alcoholism is associated with a depression of CMI, but it could be argued that fac-tors other than ethanol consumption, such as malnutrition and vitamin deficiencies, could be the major determinants of the impairment of the immune system. A direct effect of ethanol is supported by animal experiments, in which both T-lymphocyte functions and B-lymphocyte responses to T-dependent antigens are compromised after 8 days of ethanol ad-ministration.
2. Cannabinoids
There is little concrete evidence for an immunosuppressive effect of cannabinoids in hu-mans, except for depressed results in in vitro T-lymphocyte function tests and an increased incidence of herpes genitalis among young adults who use cannabinoids. In laboratory an-imals, cannabinoid administration predominantly affects T- and B-lymphocyte functions, increases the sensitivity to endotoxin, and increases the frequency of infections by intra-cellular agents, such as Listeria monocytogenes. However, the conditions of administration of these compounds to laboratory animals are rather different from the conditions sur-rounding their use as recreational drugs.
3. Opiates
Cocaine has been shown to have direct effects on human T lymphocytes in vitro, but the re-quired concentrations greatly exceed the plasma levels measured in addicts. The results of studies carried out in addicts have been contradictory.
Intravenous heroin use is associated with a high frequency of infections. In many in-stances, the infection (thrombophlebitis, soft tissue abscesses, osteomyelitis, septic arthri-tis, hepatitis B and D, and HIV) seems clearly related to the use of infected needles, but in other cases (bacterial pneumonia, tuberculosis), the infection could result from a depres-sion of the immune system. However, to date no conclusive evidence supporting a depres-sive effect of heroin over the immune system has been published
H. Immunosuppression Associated with Infections
A wide variety of infectious agents have acquired the ability to thwart the immune system in a variety of ways and, in doing so, ensuring their ability to survive in the host for at least the time necessary for their replication.
1. Bacterial Infections
Disseminated mycobacterial infections are often associated with a state of anergy. The pa-tients fail to respond to the intradermal inoculation of tuberculin and other antigens, and their in vitro lymphocyte responses to PHA and to mycobacterial antigens are depressed. The mechanisms leading to anergy are poorly understood and probably involve more than a single factor:
An increased production of IL-10 and IL-4 could reduce the activity of TH1 helper lymphocytes, thus depressing cell-mediated immunity.
Mycobacteria infect phagocytic monocytes, and intracellular infection is associated with a depression of both the antigen-presentation capacities and the ability to deliver co-stimulatory signals to T lymphocytes (for example, the expression of CD80/86 is depressed). In addition, infected monocytes/macro-phages may release nitric oxide, which inactivates lymphocytes in the proximity of the in-fected cells.
The release of soluble immunosuppressor compounds has been demonstrated for sev-eral bacteria. Several different substances, including enzymes (ribonuclease and asparagi-nase), exotoxins (such as staphylococcal enterotoxins), and other proteins, have been shown to have immunosuppressive properties, although probably their effects are limited to reducing the specific anti-infectious immune response. The staphylococcal enterotoxins are part of a group of bacterial proteins known as superantigens in vitro most superantigens have stimulatory properties, but when administered in vivo they induce generalized immunosuppression (perhaps as a consequence of indiscriminate nonspecific T-cell activation).
2. Parasitic Infections
Parasitic infections due to protozoa seem to be often associated with suppression of the im-mune response to the parasite itself. In some cases, however, there is evidence of the in-duction of a more generalized state of immunosuppression. For example, acute infections with Trypanosoma cruzi are associated with CMI depression that can be easily reproduced in laboratory animals. In both humans and experimental animals, there is a reduced ex-pression of IL-2 receptors, which can be interpreted as resulting from a downregulation of TH1 cells, either by cytokines or by suppressor compounds released by the parasite. Simi-lar mechanisms seem to account for the generalized immunosuppression observed in ex-perimental animals infected with Toxoplasma, Schistosoma, Leishmania, and Plasmodia.
3. Viral Infections
The AIDS epidemic has certainly focused our attention on the interplay between viruses and immunity. However, HIV is certainly not the only virus able to interfere with the immune system. A classical example is the development of a transitory state of anergy during the acute stage of measles, first reported by Von Pirquet in 1908. With the advent of modern immunology this observation was revisited. We now know that during the 3–4 weeks following the acute phase of measles, patients show lymphopenia, and the residual population of peripheral blood lymphocytes shows poor responses to mitogens and antigens such as PHA and Candida albicans. The cause of this state of anergy is the release of viral proteins by infected cells that have immunosuppressive properties. A viral nucleoprotein interacts with the FcγR on dendritic cells, and viral envelope glycoproteins interact with the viral hemagglutinin receptor, CD46. The combined effect of the inter-actions of the viral proteins with their cellular receptors is impaired function of dendritic cells, reduced release of IL-12, and loss of mitogen-induced and antigen-specific T-cell proliferation.
Other viruses, such as cytomegalovirus (CMV) and the rubella virus, can cause im-munosuppression. CMV mainly depresses the specific response to the virus, while the rubella virus induces a generalized immunosuppression, similar to that caused by the measles virus. Viruses can also release suppressor factors (Herpes simplex virus secretes a protein similar to IL-10, which can downregulate cytokine release by activated T lympho-cytes) and interfere with antigen presentation (adenovirus infection is associated with a de-pressed expression of MHC-I molecules). However, patients infected with these viruses do not develop generalized immunosuppression, so it seems likely that the significance of these mechanisms is mostly related to promoting conditions favorable for the persistence of the infection.
Related Topics