Chapter: Medical Immunology: AIDS and Other Acquired Immunodeficiency Diseases
Therapy - Acquired Immunodeficiency Syndrome
Therapy
No other viral disease has been the object of the intense pharmacological research that has resulted in giant strides in the management of HIV-infected patients in industrialized countries. Starting from the often-disappointing experience with single use of reverse transcrip-tase inhibitors, the addition of new drugs to our armamentarium has proven that antiretro-viral therapy can have a beneficial impact on the evolution of HIV infections.
1. Antiretroviral Agents
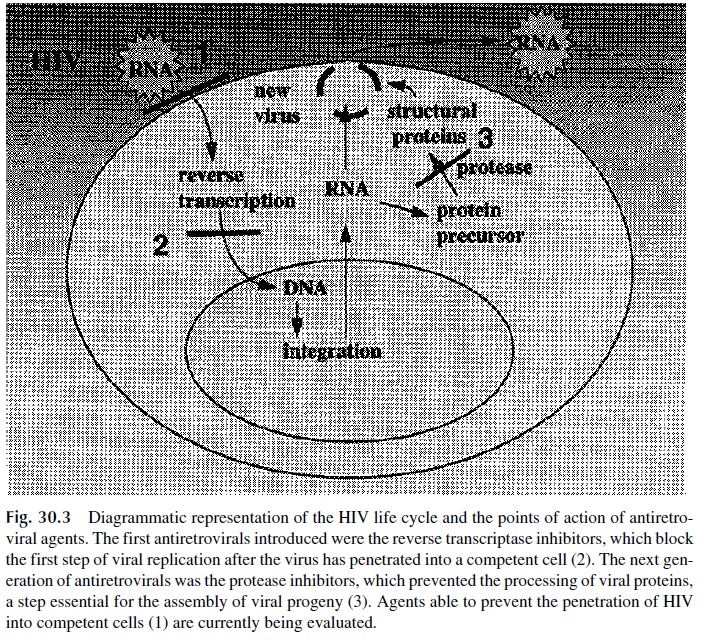
The antiretroviral agents currently in use can be divided into several different pharmaco-logical groups, acting at different levels of the viral-replication cycle (Fig. 30.3).
1. Reverse transcriptase inhibitors, which can be subclassified into three groups:
Nucleoside analogs, including zidovudine (azidodideoxythymidine, ZDV, AZT), the most widely used antiviral agent for treatment of HIV infections, and several other compounds [zalcitabine (2’ ,3’ -dideoxycytidine, ddC), didanosine (2’ ,3’ -dideoxyinosine, ddl), stavudine (2’ 3’ -didehydro-3’ deoxythymidine, d4T), lamivudine (2’ -deoxy-3’ -thiacytidine, 3TC), and abacavir (ABC)]. These compounds are phosphorylated by cellular thymidine kinases and are taken up preferentially by the HIV reverse transcriptase. DNA transcription is blocked by at least two mechanisms: binding to the reverse transcriptase, which blocks its active site, and termination of DNA synthesis when the activated nucleosides are incorporated into nascent viral DNA and block further elongation.
Nucleotide analogs, such as adefovir, are compounds that will terminate DNA elongation upon incorporation into nascent DNA chains. These drugs are still in the clinical trial stage.
Nonnucleoside reverse transcriptase inhibitors (NNRTI), such as nevirap-ine, delavirdine, and efavirenz. These compounds bind to a hydrophobic pocket of the reverse transcriptase at a site different from the active site but sill block the activity of the enzyme by steric hindrance. Because of the different binding sites, strains of HIV resistant to nucleoside reverse transcriptase inhibitors are not cross-resistant to NNRTIs.
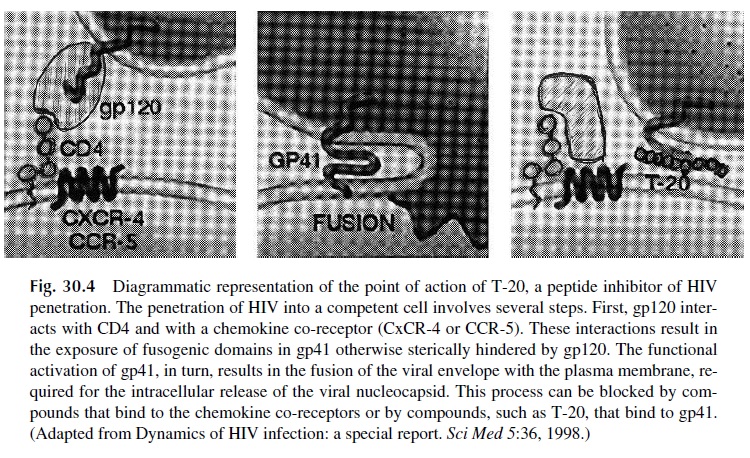
3. Protease inhibitors, such as saquinavir, ritonavir, indinavir, nelfinavir, and am-prenavir. These are synthetic, nonhydrolyzable synthetic peptides that compete as substrates for the HIV protease. HIV-infected cells exposed to these com-pounds accumulate gagpolyprotein precursors that are not cleaved due to the in-hibition of protease activity. This results in cell death. Inhibitors of viral penetration. Several compounds have been tested for the treat-ment of HIV infections by preventing HIV penetration in noninfected cells. Anti-CD4 antibodies were the first to be tried, without success. Chemokine receptor blockers and synthetic peptides such as T-20 (pentafuside), which block the in-teraction of gp41 with the plasma membrane, are under evaluation (Fig. 30.4).
4. Hydroxyurea, a ribunucleotide reductase, has been shown to have synergistic an-tiretroviral effects when given in association with ddI. Hydroxyurea inhibits the formation of deoxynucleoside-5’ -triphosphates (including dATP) required for reverse transcription of viral RNA. At the same time, ddI is converted into ddATP, which is taken up by the reverse transcriptase instead of dATP and blocks DNA chain elongation. Questions about its effectiveness and safety have yet to fully answered
Administration of three or more different antiretrovirals, known as highly active an-tiretroviral therapy (HAART), is the current standard recommendation for HIV therapy. Most combinations include two reverse transcriptase inhibitors with different binding sites in the polymerase (e.g., ZDV and 3TC). The rationale for the association is to combine nucleoside analogs that require different HIV mutations for resistance to develop. The probability of emergence of a double mutant polymerase retaining functional activity is relatively low. The third drug can be a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor.
If and when resistant strains emerge after combination therapy with two RT in-hibitors and a protease inhibitor, there are limited options left for the patient in question. For this reason some groups advocate using three RT inhibitors (two nucleoside analogs and a nonnucleoside RT inhibitor) to initiate therapy in a previously untreated patient, sav-ing the protease inhibitors for use when resistance to the initial therapy develops.
HAART in any of its modalities induces remarkable reductions in viral load after 12 weeks of administration—very frequently below the detection level of the most sensitive assays in compliant patients (the desirable goal of therapy). The reduction in viral load is usually associated with increases in CD4+ T-cell counts, averaging 120–150 over the pre-treatment levels after the first months of therapy. In addition, several immune function pa-rameters show improvement after combination therapy: skin test reactivity, mitogenic re-sponses, IFNγ and IL-2 synthesis, CD28 expression (by both CD4+ and CD8+ cells). At the same time, IL-6 and TNF levels decrease, reflecting a lower degree of T-cell activation.
The increase in CD4 T-cell numbers (affecting both 45RA and 45RO subpopula-tions) is usually not associated with an immediate increase in the repertoire of TcR Vβ re-gions. In fact, the immediate expansion of CD4 T cells seems to involve the residual mem-ory and naïve T-cell clones that survived the effects of HIV infection. Progressive, but slow, increases in CD4 counts are often seen during years. Recovery of normal T-cell counts may take 6 or more years, assuming HIV replication is suppressed during that pe-riod. It is noteworthy that when HAART succeeds in reducing the viral load to undetectable levels, HIV-specific CD4 and CD8 responses remain depressed, suggesting that specific HIV immunity not only requires regeneration of lymphocyte pools, but also antigenic ex-posure.
Some groups advocate periodic interruptions of antiretroviral therapy to allow for a burst of HIV replication, which may then induce specific immunity, but the real thera-peutic value of these protocols is yet to be established.
In spite of all these limitations, patients successfully treated with any modality of HAART are able to mount immune responses when properly immunized and show both a decrease in the frequency of opportunistic infections and a remarkable significant reduc-tion in mortality. These beneficial effects are observed even in the absence of a complete regeneration of the TcR repertoire (it has been estimated that effective immune responses can be generated with 10% of the original TcR repertoire).
Effective HAART is not associated with emergence of mutant viruses. Interruption of treatment is usually followed by the reemergence of the original viral strains character-ized before this type of therapy was initiated. In patients who develop strong anti-HIV cy-totoxic responses during HAART, suspension of therapy is not immediately followed by increase in viral load. Some of these patients have maintained undetectable HIV loads for up to 24 months after therapy interruption. Whether these cases represent examples of com-plete eradication of HIV after combination therapy has yet to be reported. There is concern that the infection of microglial cells in the brain, macrophages/dendritic cells in the lym-phoid tissues, and long-lived resting memory cells may not be efficiently eliminated by cur-rent antiretroviral therapy.
2. Indications for Antiretroviral Therapy and Problems Encountered on Its Implementation
Therapy is unquestionably indicated for symptomatic HIV-positive patients, even if not fulfilling the AIDS diagnostic criteria, and for HIV-infected patients with viral loads greater than 30,000, irrespective of CD4 counts and clinical symptoms. However, there are very many controversial areas. Some groups advocate treatment as early as possible, irre-spective of the viral load, in the hope of eradicating the virus. Others advocate treatment of all patients with CD4 counts below 300/ µL, irrespective of the viral load. On the other hand, some specialists feel that it is reasonable to withhold antiretroviral therapy in HIV-positive patients with stable CD4 counts in excess of 300/ µL and stable viral loads, below 10,000 copies/mL.
The treatment with multiple drug combinations, including those needed to treat or prevent opportunistic infections , raises serious problems, such as cost, compli-ance, and drug interactions. The fact that cost may reach tens of thousands of dollars per year is a major limiting factor worldwide, even in the United States among those patients who lack medical insurance. Compliance is made difficult not only by the number of drugs involved, but also by the complicated administration schedules. While some drugs may be taken on alternate days, others have to be taken every 4 hours. Some should be taken on an empty stomach, others after a fatty meal. Also, the side effects may be so distressing that some patients cannot tolerate the therapy.
Drug interactions become a very significant problem when multiple potent drugs are simultaneously administered. The interactions happen at many different levels. For exam-ple, not all combinations of antiretroviral drugs are adequate; some can interfere with each other and reduce the efficiency of the treatment. AZT and high doses of TMP/SMX should not be administered at the same time because both drugs can cause bone marrow depression. Some protease inhibitors inhibit the effect of tuberculostatics.
Some members of the azole group of antifungal drugs reduce the metabolic elimination of protease inhibitors. De-tailed knowledge of potential drug interactions is essential for the proper treatment of these patients.
3. Prevention of Materno-Fetal Transmission
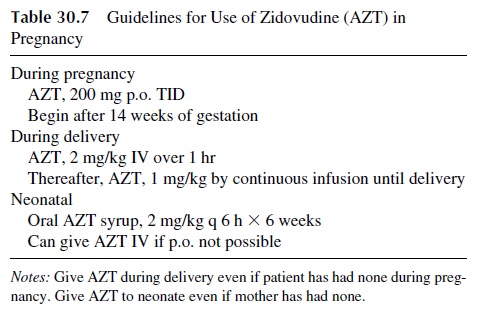
The risk of materno-fetal transmission of HIV is estimated to be around 25–30%, with a di-rect correlation with the viral load of the infected mother. The risk can be reduced by a va-riety of antiretroviral regimens that should contain ZDV (see Table 30.7). Zidovudine by itself, when give according to the guidelines shown in Table 30.6, reduces the risk of HIV transmission to about 8%. Further reductions (to about 2%) are possible by using HAART or by delivering the baby elective C-section.
4. Resistance to Antiretrovirals
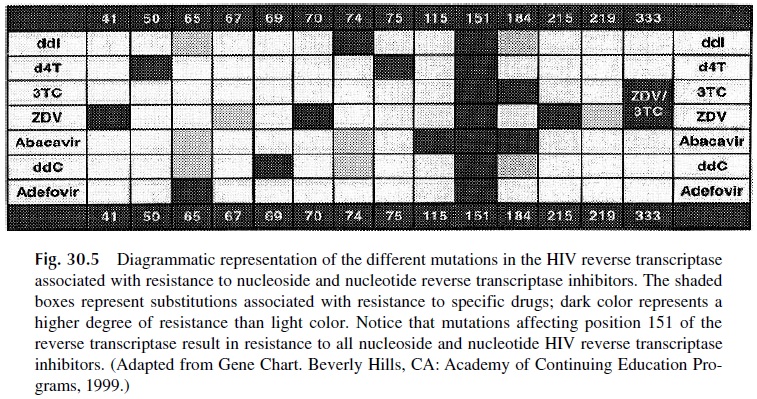
The high mutation rate of HIV associated with the selective pressure exerted by antiretro-virals results in the rapid emergence of resistant strains. The likelihood of such strains emerging is reduced when several antiretrovirals are used in combination and the patient complies with the prescribed schedule of administration. To better decide what antiretrovi-rals to use, particularly when trying to decide the most adequate combination of antiretro-virals to be given to a patient who has developed resistance, two basic methods of deter-mining antiretroviral susceptibility of HIV have been introduced. One is a phenotypic assay, based on testing the ability of HIV isolated from a given patient to replicate in per-missive cells in the presence of different antiretrovirals. The other approach is a genotypic test, which consists of analyzing the nucleic acid of a patient’s isolate to determine the pres-ence of mutations known to be associated with resistance to different antiretrovirals (Fig. 30.5). Neither approach is perfect, because the sensitivity is such that viral populations rep-resenting less than 20% of the total HIV load will not be detected.
5. Ancillary Therapies
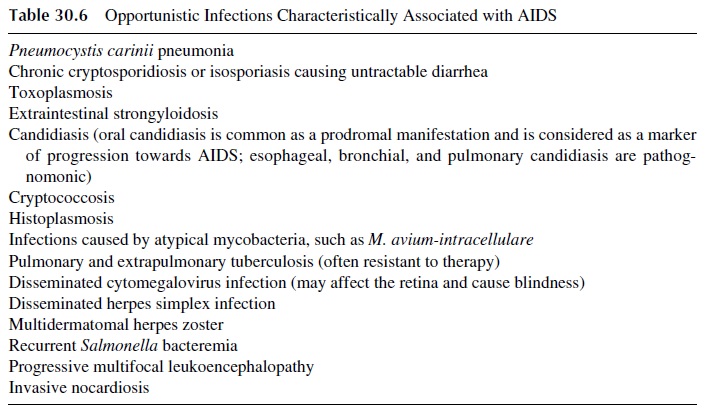
Treatment and prophylaxis of life-threatening opportunistic infections is essential and of-ten needs to take place before HAART is initiated. Treatment and prevention of infection by Pneumocystis carinii with trimethoprim-sulfamethoxazole has a major impact in the management of patients with overt AIDS.
Those patients may also benefit from chemo-prophylaxis of infections due to Toxoplasma gondii, mycobacteria, Candida albicans,Cryptococcus neoformans, and cytomegalovirus. Passive immunoprophylactic administra-tion of intravenous gamma globulin to prevent pyogenic infections is mainly indicated in infantile AIDS.
Biological response modifiers have also been widely used been used in patients with AIDS, with a variety of goals. Erythropoietin and granulocyte colony-stimulating factor (G-CSF) have been administered to patients with neutropenia or red cell aplasia secondary to the administration of ZDV to promote the proliferation of red cell and neutrophil pre-cursors. Interferon-α has been approved for administration to patients with Kaposi’s sar-coma. Interleukin-2 and interleukin-12 are being evaluated for the capacity to accelerate the restoration of immune functions in AIDS patients. High doses of IL-2 induce increases in CD4 counts in patients in which antiretrovirals have significantly reduced the viral load, but the side effects are considerable. Lower doses are better tolerated but not as effective. In either modality, the beneficial effects of IL-2 can be detected for several weeks after ad-ministration.
One of the potential adverse effects of IL-2 administration would be to activate in-fected resting T cells and promote viral replication. Recent reports from Clifford Lane and A. Fauci’s groups at the NIH suggest that this may actually allow eradication of HIV in pa-tients receiving HAART therapy. The induction of viral replication in infected cells that otherwise would remain dormant long-term reservoirs of the virus may allow their elimi-nation by the joint effects of antiretroviral compounds and the immune system.
Another avenue explored by several groups trying to boost the immune system of HIV+ individuals is the immunization of infected patients with the purpose of accelerating the immune reconstitution of patients successfully treated with HAART. Killed HIV, gp120-depleted HIV, recombinant envelope subunits, and recombinant canary pox vac-cines have been used for that purpose. These vaccines have been reported to increase thenumber of mitogen-responsive T cells in peripheral blood; the canary pox vaccine induces cytotoxic T cells in 50–70% of the recipients. Recent reports also suggest that a Tat vac-cine may be worth developing. Antibodies to the Tat protein have several potentially use-ful effects, such as reducing the level of HIV replication and transmission and preventing some of the immunosuppressive effects of the virus. In a monkey model this vaccine re-duced HIV viremia to undetectable levels and prevented CD4+ T-cell decrease.
Related Topics