Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Recombinant Coagulation Factors and Thrombolytic Agents
Reteplase - Second Generation Recombinant Thrombolytic Agents
Reteplase
Reteplase is a 355-amino acid deletion variant of t-PA, consisting of the protease and kringle 2 domains ofhuman t-PA. It is expressed in Escherichia coli cells as a single chain, non-glycosylated, 39.6-kDa peptide.
Pharmacology
Like alteplase, reteplase is a fibrin-specific activator of plasminogen. In vitro, the plasminogenolytic activity of reteplase is 2- to 3.8-fold lower than alteplase on a molar basis (Kohnert et al., 1993), which may be attributed to the absence of the finger domain in reteplase. Reteplase had a similar in vitro maximal efficacy (Emax) compared with alteplase, however, the molar concentration required to produce 50% clot lysis (EC50) was 6.4-fold higher for reteplase than for alteplase (Martin et al., 1993). The data also suggested that in vitro, reteplase has a lower thrombolytic potency in lysing aged and platelet-rich clots com-pared with alteplase.
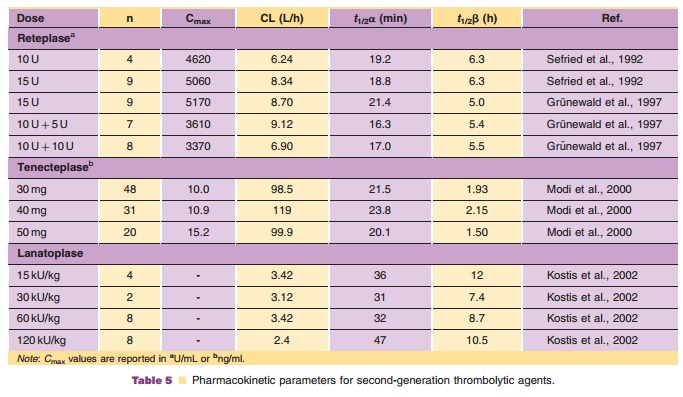
A summary of the pharmacokinetics of reteplase in humans is presented in Table 5.
Clinical Usage
Reteplase (Retavase , PDL) is indicated for use in the management of AMI in adults for the improvement of ventricular function following AMI, the reduction of the incidence of congestive heart failure, and the reduction of mortality associated with AMI.
The potency of reteplase is expressed in units using a reference standard that is specific for reteplase and is not comparable with units used for other thrombolytic agents. Reteplase is administered as a double bolus injection regimen consisting of 10 U each. Each bolus is administered as an intravenous injection of 2 minutes via an intravenous line in which no other medications are being administered simulta-neously. The second bolus injection is given 30 min-utes after the first. Heparin and reteplase are incompatible when combined in solution, and should not be administered simultaneously through the same intravenous line. If reteplase is administered through an intravenous line containing heparin, normal saline or 5% dextrose solution should be flushed through the intravenous line before and following reteplase.
The International Joint Efficacy Comparison of Thrombolytics (INJECT) trial evaluated the effects of reteplase (10 þ 10 U) and streptokinase (1.6 million Units over 60 minutes) on 35-day mortality in 6010 AMI patients in a double-blind randomized fashion. The 35-day mortality was 9.0% for patients treated with reteplase and 9.5% for those treated with streptokinase with no difference between the two groups. The incidence of stroke was also similar between the groups, however, more patients treated with reteplase experienced hemorrhagic strokes.
Two open-label angiographic studies (Reteplase Angiographic Phase II International Dose-finding study (RAPID 1) and Reteplase versus Alteplase Patency Investigation During myocardial infarction (RAPID 2)) have compared reteplase with alteplase. In RAPID 1 patients were treated with reteplase (10 þ 10 U, 15 U, or 10 þ 5 U) or the standard alteplase regimen (100 mg over 3 hours) within 6 hours of symptoms. Ninety-minute TIMI grade 3 flow was seen in 63% of patients in the 10 þ 10 U reteplase group and 49% of the patients in the standard regimen alteplase group.
RAPID 2 was an open-label, randomized trial in 320 patients comparing 10 þ 10 U reteplase and accelerated alteplase within 12 hours of symptom onset. Percentages of patients with TIMI grade 3 flow at 90 minutes were 59.9% in the reteplase group and 45.2% in the alteplase group. There was no significant difference in the 35-day mortalitybetween the two groups. Neither trial was powered to compare the efficacy or safety with respect to mortality or incidence of stroke.
The more favorable results for reteplase com-pared with alteplase noted in smaller trials were not replicated in a large, randomized, double-blind trial. In the GUSTO-III trial, 15,059 patients were rando-mized in a 2:1 fashion to receive reteplase in 2 bolus doses of 10 U 30 minutes apart or up to 100 mg alteplase infused over 90 minutes. The 30-day mortality rates were 7.47% for reteplase and 7.24% for alteplase (The Global Use of Strategies to Open Occluded Coronary Arteries ( G U S T O I I I ) Investigators, 1997). The stroke rate was 1.64% forConcerns
As with other thrombolytic agents, reteplase is contraindicated in cases of active internal bleeding, history of cerebrovascular accident, recent intracranial or intraspinal surgery or trauma, intracranial neo-plasm, arteriovenous malformation, or aneurism, in cases of known bleeding diathesis, and in severe uncontrolled hypertension.
Pharmaceutical Considerations
Reteplase is supplied as a sterile, white, lyophilized powder for intravenous injection after reconstitution with sterile water for injection, USP supplied as part of the kit. Following reconstitution, the pH of the solution is 6.0. Reteplase contains no antibacterial preservatives and should be reconstituted immedi-ately before use. The solution should be used within 4 hours when stored at 2 C to 30 C (36–86 F).
Related Topics