Chapter: Medical Physiology: Regulation of Acid-Base Balance
Respiratory Regulation of Acid-Base Balance
Respiratory Regulation of Acid-Base Balance
The second line of defense against acid-base disturbances is control of extracellular fluid CO2 concentration by the lungs. An increase in ventilation eliminates CO2 from extracellular fluid, which, by mass action, reduces the H+ concentration. Conversely, decreased ventilation increases CO2, thus also increasing H+ concentration in the extracellular fluid.
Pulmonary Expiration of CO2 Balances Metabolic Formation of CO2
CO2 is formed continually in the body by intracellular metabolic processes. After it is formed, it diffuses from the cells into the interstitial fluids and blood, and the flowing blood transports it to the lungs, where it dif-fuses into the alveoli and then is transferred to the atmosphere by pulmonary ventilation. About 1.2 mol/ L of dissolved CO2 normally is in the extracellular fluid, corresponding to a PCO2 of 40 mm Hg.
If the rate of metabolic formation of CO2 increases, the PCO2 of the extracellular fluid is likewise increased. Conversely, a decreased metabolic rate lowers the PCO2. If the rate of pulmonary ventilation is increased, CO2 is blown off from the lungs, and the PCO2 in the extracellular fluid decreases. Therefore, changes in either pulmonary ventilation or the rate of CO2 for-mation by the tissues can change the extracellular fluid PCO2.
Increasing Alveolar Ventilation Decreases Extracellular Fluid Hydrogen Ion Concentration and Raises pH
If the metabolic formation of CO2 remains constant, the only other factor that affects PCO2 in extracellular fluid is the rate of alveolar ventilation. The higher the alveolar ventilation, the lower the PCO2; conversely, the lower the alveolar ventilation rate, the higher the PCO2. As discussed previously, when CO2 concentration increases, the H2CO3 concentration and H+ concentra-tion also increase, thereby lowering extracellular fluid pH.
Figure 30–2 shows the approximate changes in blood pH that are caused by increasing or decreasing the rate of alveolar ventilation. Note that increasing alveolar ventilation to about twice normal raises the pH of the extracellular fluid by about 0.23. If the pH of the body fluids is 7.40 with normal alveolar ventila-tion, doubling the ventilation rate raises the pH to
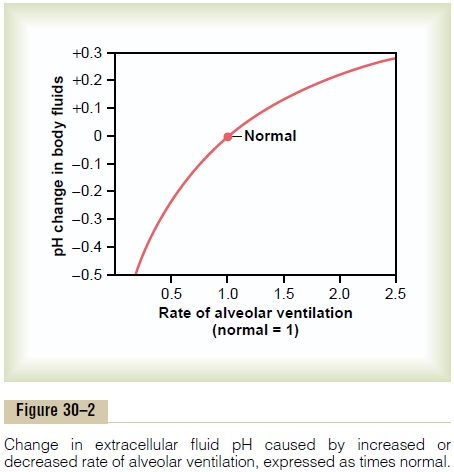
about 7.63. Conversely, a decrease in alveolar ventila-tion to one fourth normal reduces the pH by 0.45. That is, if the pH is 7.4 at a normal alveolar ventilation, reducing the ventilation to one fourth normal reduces the pH to 6.95. Because the alveolar ventilation rate can change markedly, from as low as 0 to as high as 15 times normal, one can easily understand how much the pH of the body fluids can be changed by the respira-tory system.
Increased Hydrogen Ion Concentration Stimulates Alveolar Ventilation
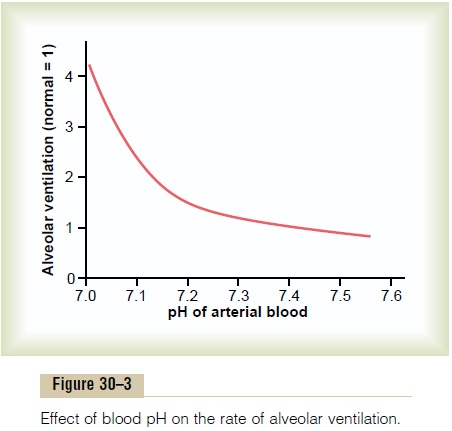
Not only does the alveolar ventilation rate influence H+ concentration by changing the PCO2 of the body fluids, but the H+concentration affects the rate of alveolar ventilation. Thus, Figure 30–3 shows that the alveolar ventilation rate increases four to five times normal as the pH decreases from the normal value of 7.4 to the strongly acidic value of 7.0. Conversely, when plasma pH rises above 7.4, this causes a decrease in the ventilation rate. As one can see from the graph, the change in ventilation rate per unit pH change is much greater at reduced levels of pH (corresponding to ele-vated H+ concentration) compared with increased levels of pH. The reason for this is that as the alveolar ventilation rate decreases, owing to an increase in pH (decreased H+ concentration), the amount of oxygen added to the blood decreases and the partial pressure of oxygen (PO2) in the blood also decreases, which stimulates the ventilation rate. Therefore, the respira-tory compensation for an increase in pH is not nearly as effective as the response to a marked reduction in pH.
Feedback Control of Hydrogen Ion Concentration by the Respi-ratory System. Because increased H+concentrationstimulates respiration, and because increased alveolar ventilation decreases the H+ concentration, the respi-ratory system acts as a typical negative feedback con-troller of H+ concentration.
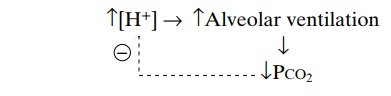
That is, whenever the H+ concentration increases above normal, the respiratory system is stimulated, and alveolar ventilation increases. This decreases the PCO2 in extracellular fluid and reduces H+ concentra-tion back toward normal. Conversely, if H+concen-tration falls below normal, the respiratory center becomes depressed, alveolar ventilation decreases, and H+ concentration increases back toward normal.
Efficiency of Respiratory Control of Hydrogen Ion Concen- tration. Respiratory control cannot return the H+concentration all the way back to normal when a dis-turbance outside the respiratory system has altered pH. Ordinarily, the respiratory mechanism for con-trolling H+concentration has an effectiveness between 50 and 75 per cent, corresponding to a feedback gain of 1 to 3. That is, if the H+concentration is suddenly increased by adding acid to the extracellular fluid and pH falls from 7.4 to 7.0, the respiratory system can return the pH to a value of about 7.2 to 7.3. This response occurs within 3 to 12 minutes.
Buffering Power of the Respiratory System. Respiratory reg-ulation of acid-base balance is a physiologic type of buffer system because it acts rapidly and keeps the H+concentration from changing too much until the slowly responding kidneys can eliminate the imbalance. In general, the overall buffering power of the respiratory system is one to two times as great as the buffering power of all other chemical buffers in the extracellu-lar fluid combined. That is, one to two times as much acid or base can normally be buffered by this mecha-nism as by the chemical buffers.
Impairment of Lung Function Can Cause Respiratory Acidosis. We have discussed thus far the role of the normal res-piratory mechanism as a means of buffering changes in H+ concentration. However, abnormalities of respi-ration can also cause changes in H+concentration. Forexample, an impairment of lung function, such as severe emphysema, decreases the ability of the lungs to eliminate CO2; this causes a buildup of CO2 in the extracellular fluid and a tendency toward respiratoryacidosis. Also, the ability to respond to metabolic aci-dosis is impaired because the compensatory reduc-tions in PCO2 that would normally occur by means of increased ventilation are blunted. In these circum-stances, the kidneys represent the sole remaining phys-iologic mechanism for returning pH toward normal after the initial chemical buffering in the extracellular fluid has occurred.
Related Topics